Rep:Mod:MJW11
Mike's computational chemistry wiki - MJW11
Module 1
The hydrogenation of cyclopentadiene dimer
| Exo cyclopentadiene dimer | Endo cyclopentadiene dimer | ||||
|---|---|---|---|---|---|
|
|
|
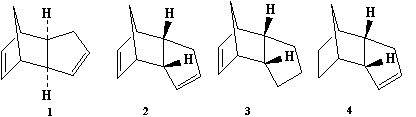
Cyclopentadiene readily undergoes dimerisation at room temperature. The dimerisation of cyclopentadiene is a Diels-Alder cycloaddition reaction. The endo isomer of cyclopentadiene is found to form preferentially over the exo isomer. An investigation into this regioselectivity is presented here, making use of molecular mechanics computations. The two possible dihydro-cyclopentadiene dimer derivatives were subjected to the same computational treatment to glean an understanding of their energetic stabilities too.
Diagram 1 - exo and endo cyclopentadiene dimers and the two possible dihydro derivatives of the endo isomer[1]
The endo and exo forms of the cyclopentadiene dimer and the two possible dihydro derivatives of the endo dimer were studied using ChemBio3D. The energies of the dimers were minimised using the Allinger MM2 molecular mechanics models.
Energy minimisation (Allinger MM2 molecular mechanics models) results
| Dimer 1 | Dimer 2 | Dimer 3 | Dimer 4 | |
|---|---|---|---|---|
| Total energy / kcalmol-1 | 31.8764 | 33.9975 | 34.9643 | 29.2475 |
| Stretch | 1.2846 | 1.2502 | 1.2488 | 1.1300 |
| Bend | 20.5807 | 20.8479 | 19.1569 | 13.0132 |
| Stretch-Bend | -0.8380 | -0.8357 | -0.8352 | -0.5653 |
| Torsion | 7.6556 | 9.5109 | 11.0763 | 12.4121 |
| Non-1,4 VDW | -1.4175 | -1.5439 | -1.6412 | -1.3246 |
| 1,4 VDW | 4.2335 | 4.3205 | 5.7965 | 4.4411 |
| Dipole - dipole | 0.3775 | 0.4476 | 0.1622 | 0.1410 |
The results of the energy minimisation show that the exo cyclopentadiene dimer has a lower total energy than the endo dimer by 2.1211 kcalmol-1. The exo cyclopentadiene dimer is therefore more energetically stable than the endo one. However, a greater proportion of the endo dimer is formed during the dimerisation of cyclopentadiene which implies that the reaction takes place under kinetic control as opposed to thermodynamic control.[1] Although the energy of the exo dimer is lower than the energy of the endo dimer, the transition state passed through during the production of the endo dimer must be lower in energy than that for the exo dimer.
The main difference in the energies of the two diastereomers is the greater torsion energy of the endo isomer. This energetic difference can be rationalised on the basis of the closer proximity of the two larger carbon rings in the endo isomer than in the exo dimer which results in a greater torsional strain.
The lower energy of the endo cyclopentadiene dimer transition state is thought to be due to a more favourable HOMO - LUMO interaction than for the exo dimer. The two cyclopentadiene molecules are thought to lie on top of one another in the transition state for the formation of the endo dimer with a subsequent 'unfolding' of the system towards the products through the reaction coordinate. The two cyclopentadiene molecules are displaced from one another in the transition state for the exo dimer which leads to poorer HOMO - LUMO interaction and thus a higher energy transition state. Calculations of the relative energies of the transition states in this dimerisation reaction would require molecular orbital treatment to be sufficiently rigorous, such a treatment is beyond the scope of this investigation.
Dimeric and monomeric cyclopentadiene exist in equilibrium. The conclusion that the dimerisation takes place under kinetic control could be verified by performing the reaction at different temperatures and leaving the reaction to take place for different periods of time: higher temperatures and longer reaction times should increase the proportion of the exo dimer in the mixture.
Dimer 4 has a lower total energy than dimer 3 by 5.7168 kcalmol-1 The main reason for this difference in total energy appears to be the difference in the bend energies of the two dihydro derivatives. Dimer 3 shows a greater bend energy due to the remaining carbon carbon double bond being more strained than in dimer 4. Carbon atoms in carbon carbon double bonds are sp2 hybridised for which optimal bond angles are 120o. The bond angles about the carbon carbon double bond in dimer 3 are further from 120o (less) than in dimer 4 due to greater ring strain in dimer 3.
Stereochemistry and reactivity of an intermediate in the synthesis of Taxol
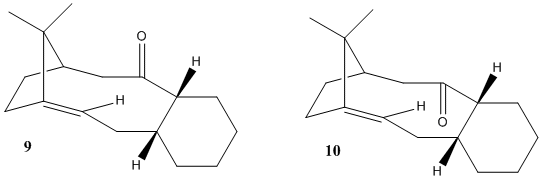
A total synthesis of Taxol, a drug used for the treatment of ovarian cancer, proposed by Paquette involves an intermediate which exhibits atropisomerism. On standing, the two atropisomers interconvert.[2] The aim of this investigation was to find which of the two atropisomers is more stable and also to work out why the intermediate becomes significantly less reactive after alkene hydrogenation.[2] The two atropisomers of the intermediate are shown in Diagram 2.
The atropisomers were modelled using ChemBio3D and their energies were calculated using the Allinger MM2 molecular mechanics models as before. Their energies were calculated using the MMFF94 molecular mechanics models too.
Energy minimisation (Allinger MM2 molecular mechanics models) results
| Compound 9 - chair conformation | Compound 10 - chair conformation | Compound 9 - twist-boat conformation | Compound 10 - twist-boat conformation | |
|---|---|---|---|---|
| Total energy / kcalmol-1 | 47.8396 | 42.6830 | 53.3048 | 57.6481 |
| Stretch | 2.7831 | 2.6213 | 2.9466 | 3.3491 |
| Bend | 16.5363 | 11.3392 | 17.2054 | 17.3168 |
| Stretch-Bend | 0.4301 | 0.3439 | 0.5008 | 0.4572 |
| Torsion | 18.2566 | 19.6658 | 21.2910 | 23.4675 |
| Non-1,4 VDW | -1.5543 | -2.1568 | -1.4137 | -0.2708 |
| 1,4 VDW | 13.1124 | 12.8717 | 14.5051 | 15.1083 |
| Dipole - dipole | -1.7245 | -2.0022 | -1.7304 | -1.7801 |
Energy minimisation (MMFF94 molecular mechanics models) results
| Compound 9 - chair conformation | Compound 10 - chair conformation | Compound 9 - twist-boat conformation | Compound 10 - twist-boat conformation | |
|---|---|---|---|---|
| Total energy / kcalmol-1 | 70.5436 | 60.5670 | 77.9325 | 81.5249 |
Compound 10 with its cyclohexane ring in the chair conformation has the lowest total energy as calculated using the MM2 models and the MMFF94 models. It is therefore expected that this atropisomer would predominate in a mixture and that attack of the carbonyl group would occur most frequently via this conformation. Interestingly, compound 10 appears to have a higher energy than compound 9 when both have their cyclohexane rings in the twist boat conformation using both the MM2 and the MMFF94 models.
Atropisomers 9 and 10 are examples of hyperstable olefins: systems in which the alkene is less strained than the corresponding alkane. Olefin strain energy is defined as the difference between the strain energy of an olefin and its corresponding saturated hydrocarbon.[3] Allinger MM2 molecular models were used to compare the energies of compound 10 in the chair conformation and its corresponding saturated hydrocarbon. The olefin strain energy was found to be -8.43 kcalmol-1. The origin of the stabilising olefin strain energy is thought to be the relevant carbon carbon bond preferring a bond angle closer to 120o (sp2) than 109.5o (sp3) as is the case in this molecule due to steric reasons.
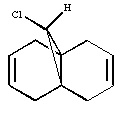
Dichlorocarbene reacts with electrophiles in addition reactions. Molecular orbital calculations were used here in order to adequately rationalise the nucleophilicity of each alkene bond in compound 12, molecular mechanics considerations being insufficient for such a task.
The energy of Compound 12 was minimized using ChemBio3D (Allinger MM2 molecular mechanics models) in order to prepare the conformation of the molecule for calculation with the MOPAC method. An approximate representation of the valence electron - molecular wavefunction was calculated using the MOPAC method and contour plots of the resulting molecular orbitals are presented below.
N.B Compound 12 has a plane of symmetry which bisects the two alkene bonds and is perpendicular to the plane of the bicyclic system. However, these MOs don't show such a symmetry. This is potentially worrying for the accuracy of the molecular orbital arguments presented here.
The highest occupied molecular orbital contour plot shows lobes close to the alkene bond closest to the chlorine atom and no lobes close to the other alkene bond. The contour plot provides an indication of electron density as the square of the wavefunction is a probability density function. The HOMO contour plot therefore suggests that the alkene bond closest to the chlorine atom has a greater electron density than the other alkene bond. Therefore, according to this calculation, electrophiles are more likely to attack the alkene bond closest to the chlorine atom in an electrophillic addition reaction (ignoring steric effects).
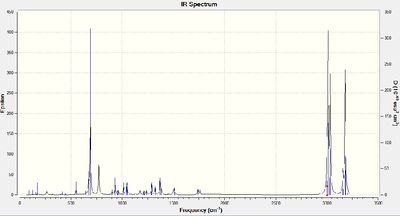
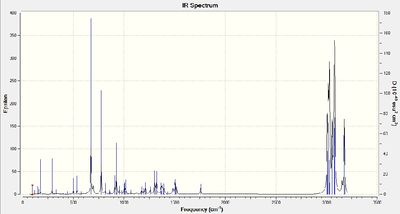
Infrared spectra were produced for compound 12 and its dihydro derivative (hydrogenation of alkene furthest from the chlorine atom) by using the density functional approach with Gaussian.
Stretching frequencies
C - Cl: 766 cm-1
C=C (closest to Cl): 1757 cm-1
C=C (furthest from Cl): 1747 cm-1
Stretching frequencies
C - Cl: 770 cm-1
C=C (closest to Cl): 1754 cm-1
Figures 1 and 2 show that there are two alkene peaks in the spectrum for compound 12 and only one in the spectrum for the monoalkene, as would be expected. The C-Cl stretching frequency for the dialkene is lower than that for the monoalkene. This can be rationalised on the basis of there being greater C-C π to C-Cl σ * interactions in the dialkene (there are two alkene bonds as opposed to one in the monoalkene). This stronger interaction weakens the C-Cl bond in the dialkene, decreasing its stretching frequency.
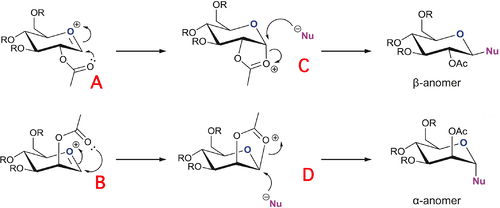
Diagram 4 shows two possible reactions of the glycosylation of a saccharide with a nucleophile leading to the formation of two stereoisomers. The aim of this investigation was to calculate the energies of the relevant species in the reactions shown to gain a better understanding of neighbouring group effects (acyl group in this instance) in glycosylation reactions. The methyl group was chosen for the R groups in order to make the calculations as simple as possible while remaining realistic, -OMe is a common protecting group in carbohydrate synthesis. R = H was avoided because of potentially unwanted hydrogen bonding interactions between hydroxyl groups which wouldn't be present in a feasible synthesis.
Energy minimisation results (Allinger MM2 molecular models)
| Compound A - acyl group above oxonium ion plane | Compound A - acyl group below oxonium ion plane | Compound B - acyl group above oxonium ion plane | Compound B - acyl group below oxonium ion plane | |
|---|---|---|---|---|
| Total energy / kcalmol-1 | 31.7909 | 18.4877 | 31.8881 | 23.4520 |
| Stretch | 2.5226 | 2.3211 | 2.2455 | 2.1735 |
| Bend | 16.0002 | 12.0440 | 13.8352 | 10.0449 |
| Stretch-Bend | 0.9579 | 0.9108 | 0.9122 | 0.7772 |
| Torsion | 3.4988 | 2.3778 | 2.3640 | 3.4782 |
| Non-1,4 VDW | -1.0400 | -1.2025 | -1.8541 | -2.3642 |
| 1,4 VDW | 19.8351 | 19.4955 | 20.2873 | 20.5721 |
| Charge - dipole | -19.1756 | -23.9692 | -13.2581 | -15.5510 |
| Dipole - dipole | 9.1920 | 6.5102 | 7.3562 | 4.3213 |
Energy minimisation results (MOPAC/PM6 method)
| Compound A - acyl group above oxonium ion plane | Compound A - acyl group below oxonium ion plane | Compound B - acyl group above oxonium ion plane | Compound B - acyl group below oxonium ion plane | |
|---|---|---|---|---|
| Heat of formation / kcalmol-1 | -71.20230 | -85.04766 | -64.08225 | -72.55941 |
| RMS Gradient | 0.09478 | 0.07678 | 0.09937 | 0.08678 |
The energy minimisation results using each of the different methods (MM2 & PM6) show the same trends. The results show that for compounds A and B, the total energy of each molecule is lower if the acyl group is pointing below the molecular plane. Importantly, compound A with the acyl group below the plane of the molecule has the lowest total energy of all the saccharides studied here.
The most important contributions to the trends presented here appear to be lower bend energies and dipole - dipole energies in the conformers with the acyl group pointing below the molecular plane. The origins of these lower energies can be seen in the models of the compounds (links in column titles).
| Energy minimisation results (Allinger MM2 molecular models) | Energy minimisation results (MOPAC/PM6) | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
In this case, the MM2 and PM6 molecular models calculations produced conflicting results about which of the compounds, C and D, has the lowest total energy. The MM2 molecular models calculations suggest that compound C has the lower energy and the PM6 molecular models calculations suggest that compound D has the lower energy. The PM6 results for these saccharides are likely to be more accurate than the MM2 results because the Allinger MM2 molecular models don't take electronic effects into account which are critical for this investigation. In fact, the anomeric effect cannot be explained in terms of steric considerations alone, the effect is stereoelectronic in nature. The MOPAC/PM6 models are better for a more complete steric / stereoelectronic treatment as needed here. Therefore, compound D is likely to have the lower total energy of the two intermediates, C and D.
Mini-project - Investigation into the regioselectivity of the Baeyer-Villiger reaction
The Baeyer-Villiger reaction is the reaction between a carbonyl compound and a peracid (commonly m-CPBA) to form an ester. Unsymmetrical carbonyl compounds as Baeyer-Villiger reagents give rise to regioisomeric products. Cyclic ketones can be usefully converted into lactones using the Baeyer-Villiger reaction. The reaction of cyclic ketones to form lactones increases ring size by one oxygen atom which acts as a thermodynamic driving force for strained systems. A cyclic system is studied here, involved in the synthesis of Kainic acid.
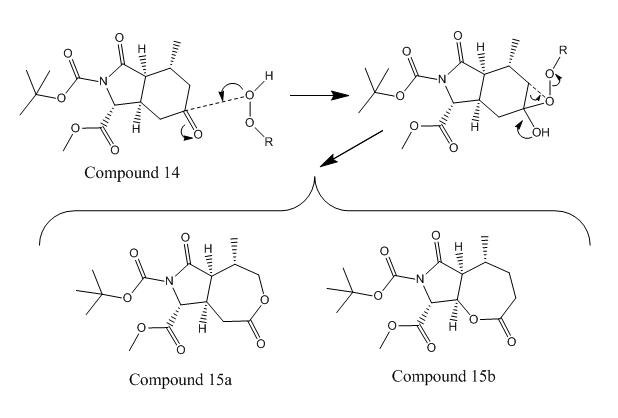
Diagram 5 - Reaction scheme showing the Baeyer-Villiger reaction of compound 14 to form the two possible regioisomers. (R = meta-chlorophenyl for m-CPBA.)
Two regioisomeric products are possible from the Baeyer-Villiger reaction of compound 14 as shown by diagram 5. The regioselectivity of Baeyer-Villiger reactions is usually rationalised on the basis of migrating group ability to stabilise positive charge.[4] In the reaction presented here, compound 15a was found to form almost exclusively. The regioselective preference of compound 15a over 15b in this reaction cannot be adequately rationalised on the basis of positive charge stabilisation by the migrating group, molecular conformation was alluded to as an explanation by Jonathan Clayden, Christel J. Menet and Kirill Tchabanenko.[5] The aim of this project was to test the authors' assertion that compound 15a was indeed the isomer formed in their synthesis and, time-permitting, that the regioselectivity is due to molecular conformation by using computational chemistry techniques.
The energies of compounds 15a and 15b were minimised using the MOPAC method and 13C NMR spectra were computed using the GIAO method.
| Compound 15a | Compound 15b | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
The energy minimisation (Allinger MM2 molecular mechanics models) results show that compound 15a has a lower total energy than compound 15b which suggests that compound 15a is more thermodynamically stable, i.e 15a is the thermodynamic product of the reaction. The difference in total energy between the two isomers is relatively large (23.00 kcalmol-1). The main contributions to this large total energy difference appear to be bend energy, torsion energy, charge - dipole energy and dipole - dipole energy - each being less energetically favourable in compound 15b. The origins of these different energy contributions can be seen in the molecular models for each compound presented above.
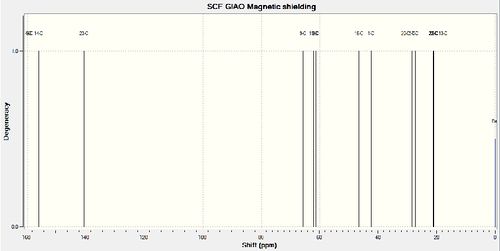
Figure 3 - Gaussian calculated 13C NMR spectrum for compound 15a
Figure 4 - Gaussian calculated 13C NMR spectrum for compound 15b
Literature 13C NMR chemical shifts for compound 15a[5] (75MHz, CDCl3) / ppm
171.5, 171.3, 169.7, 149.2, 84.4, 68.9, 63.0, 52.9, 48.7, 36.5, 32.5, 29.8, 27.8, 15.5
Gaussian calculated NMR chemical shifts for compound 15a (75MHz, CDCl3) / ppm
175.58, 167.74, 166.94, 161.58, 160.73, 155.55, 153.52, 143.87, 139.52, 130.82, 126.05, 124.77, 47.73, 29.37, 29.00, 28.39
Gaussian calculated NMR chemical shifts for compound 15b (75MHz, CDCl3) / ppm
159.79, 159.14, 156.30, 140.69, 65.75, 62.08, 61.28, 46.54, 42.41, 30.62, 28.37, 27.35, 21.20, 20.97, 20.49, 17.79
Unfortunately, 13C NMR chemical shifts were not reported for compound 15b (presumably because only compound 15a formed in their synthesis, as reported).[5]
14 13C NMR peaks were reported in the literature for compound 15a and 16 were calculated using the GIAO method in Gaussian. Compound 15a contains 16 carbon atoms and would thus be expected to show 16 peaks in its 13C NMR spectrum. Some of the peaks in the calculated NMR spectrum for compound 15a are very close together so it's possible these peaks could not be resolved in the literature NMR spectrum.
The calculated NMR spectra for compounds 15a and 15b are qualitatively similar, as would be expected. However, the chemical shifts for compound 15a are closer to those reported in the literature. e.g 171.5/175.58 vs 171.5/159.79, 171.3/167.74 vs 171.3/159.14 etc. This greater similarity between the literature spectrum and the calculated spectrum for 15a as opposed to 15b suggests that compound 15a was in fact synthesised in the authors' synthesis of Kainic acid.
Cathleen M. Crudder, Austin C. Chen and Larry A. Calhoun list two stereoelectronic requirements for the Baeyer-Villiger reaction: anti-periplanar alignment of the migrating substituent and the O-O bond of the leaving group (primary effect) and anti-periplanar alignment of the oxygen lone pair and the migrating substituent (secondary effect).[6] Molecular mechanics modelling and molecular orbital calculations for the intermediate of compound 15 formation from compound 14 would be required to investigate the relevence of the stereoelectronic requirements for this system.
Conclusion
The Gaussian calculated NMR 13C NMR spectra suggest that compound 15a was formed in Jonathan Clayden, Christel J. Menet and Kirill Tchabanenko's synthesis of Kainic acid as opposed to compound 15b, the other possible regioisomer in the Baeyer-Villiger reaction step of their synthesis. From a thermodynamic perspective, the regioselectivity of the Baeyer-Villiger reaction has been rationalised for this synthesis. However, further computations are required for analysis of the kinetic side to the reaction.
References
1. http://wiki.ch.ic.ac.uk/wiki/index.php?title=Mod:organic
2. S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319;DOI:10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0
3. Eric V. Anslyn and Dennis A. Dougherty, Modern physical organic chemistry, 2006, 139
4. Clayden, Greeves, Warren and Wothers, Organic Chemistry, 2008, 992-995
5. Jonathan Clayden, Christel J. Menet and Kirill Tchabanenko, Tetrahedron, 2002, 4727-4733
6. Cathleen M. Crudder, Austin C. Chen and Larry A. Calhoun, Angew. Chem. Int. Ed., 2000, 2851