Rep:Mod:MC11296
Year 2 Inorganic Computational Lab
BH3 Section
B3LYP/6-31G(d,p)
Item Value Threshold Converged?
Maximum Force 0.000193 0.000450 YES
RMS Force 0.000126 0.000300 YES
Maximum Displacement 0.000764 0.001800 YES
RMS Displacement 0.000500 0.001200 YES
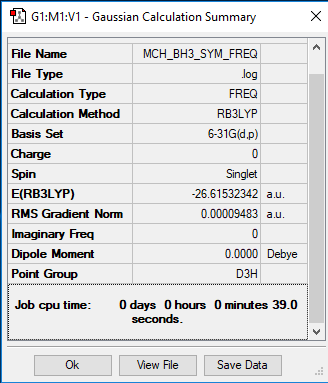
Frequency file: MCH_BH3_SYM_FREQ.LOG
Low frequencies --- -0.2260 -0.1035 -0.0054 48.0278 49.0875 49.0880 Low frequencies --- 1163.7224 1213.6715 1213.6741
Optimised BH3 Molecule |
Vibrational spectrum for BH3
| wavenumber (cm-1) | Intensity (arbitrary units) | symmetry | IR active | type |
| 1164 | 93 | A2" | yes | out-of-plane bend |
| 1214 | 14 | E' | yes | in-plane bend |
| 1214 | 14 | E' | yes | in-plane bend |
| 2580 | 0 | A1' | no | symmetric stretch |
| 2713 | 126 | E' | yes | asymmetric stretch |
| 2713 | 126 | E' | yes | asymmetric stretch |
IR Spectrum of BH3
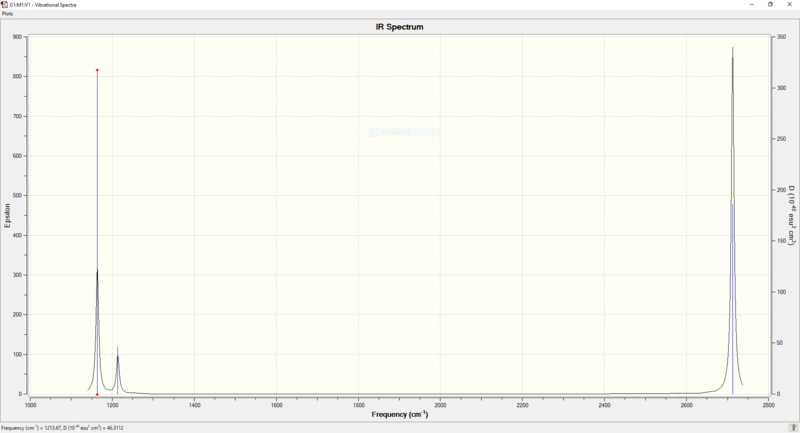
Why does the IR spectrum have less than 6 peaks, even though there are 6 vibrations?
There are six vibrations measured in Gauss View. However, only three vibrational peaks are observed in the IR spectrum of BH3. The A2" 1164 vibration is an IR active, out-of-plane bend. The two E' 1214 vibrations are IR active bends, they overlap as one peak because they have the same energy and symmetry and are therefore degenerate. The two E' 2713 vibrations are IR active asymmetric stretches, they overlap as one peak because they have the same energy and symmetry and are therefore degenerate.
The A1' 2580 vibration is IR inactive as it is a symmetric stretch (no net dipole - IR inactive).
Full information given in the table for the vibrational analysis and great explanation, clearly identifying the modes, as to why only 3 peaks are visible. Side note: Gaussian is the program which does the calculations (so 'measures' the vibrations) and GaussView is just a GUI used to view the calculation results and structures. Smf115 (talk) 21:37, 18 May 2019 (BST)
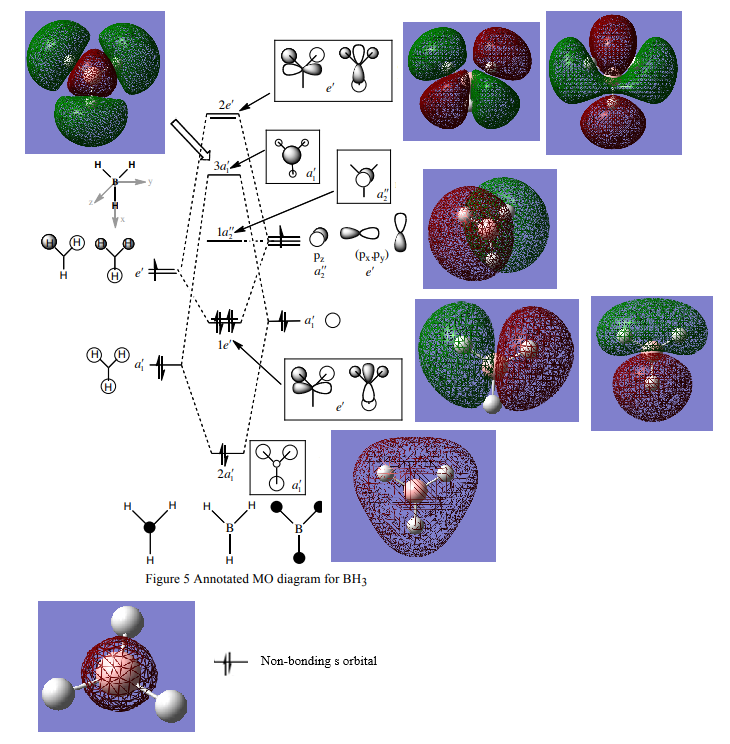
MO Diagram of BH3 and calculated MOs[1]
Are there any significant differences between the real and LCAO MOs?
What does this say about the accuracy and usefulness of qualitative MO theory?
There are no significant changes between the real and LCAO MOs, as they resemble each other and the real MOs can be easily identified by the LCAO MOs. The only significant difference is the real a1' MO, which is more spread out than the LCAO MO; however, this difference can be expected. Hence, qualitative MO theory is relatively useful and accurate.
Good inclusion of all the calculated MOs with the corresponding LCAO MO on the diagram and great reference to the differences shown by the (3!)a1' orbital. To improve, 'diffuse' is a better term than spread out and in the future, it is easier to visualise the MOs clearly with the translucent surface rather than a mesh (NB:you were not marked down for these though!) Smf115 (talk) 21:41, 18 May 2019 (BST)
Association energies: Ammonia-Borane
NH3
B3LYP/6-31B level
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000014 0.001800 YES
RMS Displacement 0.000009 0.001200 YES
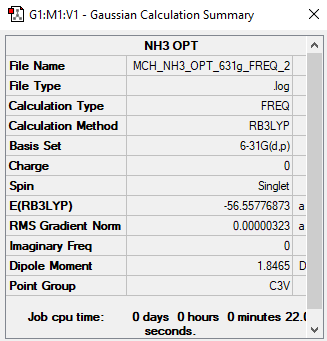
Frequency file: MCH_NH3_OPT_631G_FREQ_2.LOG
Low frequencies --- -0.0129 -0.0014 0.0018 7.1032 8.1047 8.1050 Low frequencies --- 1089.3834 1693.9368 1693.9368
Optimised NH3 Molecule |
NH3BH3
B3LYP/6-31B level
Item Value Threshold Converged?
Maximum Force 0.000208 0.000450 YES
RMS Force 0.000056 0.000300 YES
Maximum Displacement 0.001051 0.001800 YES
RMS Displacement 0.000430 0.001200 YES
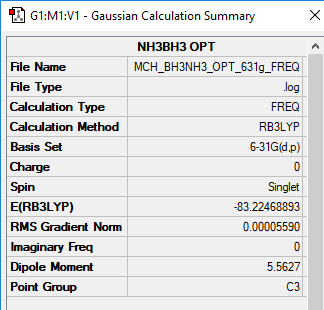
Frequency file: MCH_BH3NH3_OPT_631G_FREQ.LOG
Low frequencies --- -19.2719 -0.0311 -0.0059 0.0258 9.2807 9.2891 Low frequencies --- 262.4305 631.2241 637.8391
Optimised NH3BH3 Molecule |
Determination of bond energy: ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]= -83.22469-(-56.55777+(-26.61532))=-0.05160 au =135 kJ/mol
Is the B-N dative bond weak, medium or strong?
In comparison to a C-H bond, which is around 411kJ/mol, the B-N dative bond is relatively weak.[2]
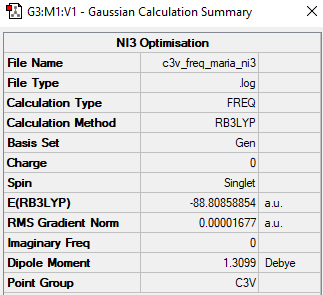
NI3
B3LYP/6-31B level
Item Value Threshold Converged? Maximum Force 0.000041 0.000450 YES RMS Force 0.000017 0.000300 YES Maximum Displacement 0.000649 0.001800 YES RMS Displacement 0.000257 0.001200 YES
Frequency file: C3v freq maria ni3.log
Low frequencies --- -12.5499 -12.5436 -5.9292 -0.0040 0.0191 0.0672 Low frequencies --- 100.9757 100.9765 147.3140
Optimised NI3 Molecule |
The optimised N-I distance is 2.184 Angstrom.
Project: Ionic Liquids
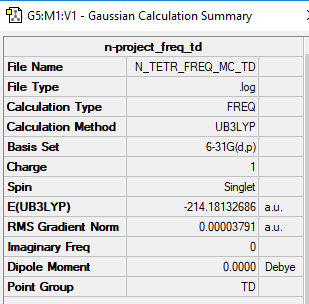
[N(CH3)4]+
B3LYP/6-31B level
Item Value Threshold Converged? Maximum Force 0.000062 0.000450 YES RMS Force 0.000038 0.000300 YES Maximum Displacement 0.000429 0.001800 YES RMS Displacement 0.000220 0.001200 YES
Frequency file: N_TETR_FREQ_MC_TD.LOG
Low frequencies --- -0.0013 -0.0012 -0.0010 35.2657 35.2657 35.2657 Low frequencies --- 217.1664 316.3173 316.3173
Optimised [N(CH3)4]+ Molecule |
[P(CH3)4]+
B3LYP/6-31B
Item Value Threshold Converged? Maximum Force 0.000012 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000060 0.001800 YES RMS Displacement 0.000052 0.001200 YES
Frequency file: P_TETR_MC_TD_FREQ.LOG
Low frequencies --- -0.0022 -0.0018 -0.0015 24.5223 24.5223 24.5224 Low frequencies --- 160.0774 194.8186 194.8186
Optimised [P(CH3)4]+ Molecule |
Charge Distribution
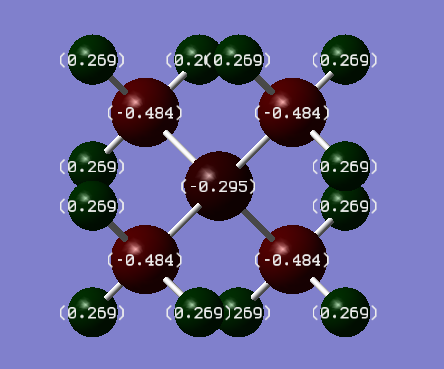
[N(CH3)4]+
Table of the the charge distribution of [N(CH3)4]+.
| Atom | Charge |
|---|---|
| Hydrogen | 0.269 |
| Carbon | -0.484 |
| Nitrogen | -0.295 |
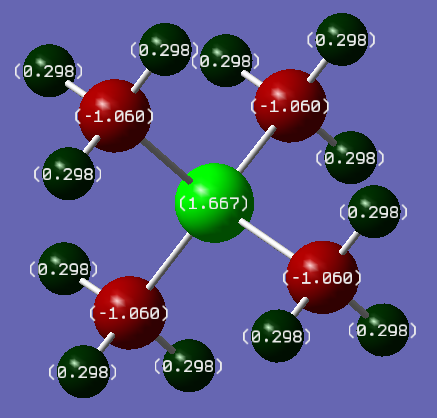
[P(CH3)4]+
Table of the the charge distribution of [P(CH3)4]+.
| Atom | Charge |
|---|---|
| Hydrogen | 0.298 |
| Carbon | -1.060 |
| Phosphorus | 1.667 |
Analysis
As the charge distribution of [N(CH3)4]+ ranged over 0.269 to -0.484 and of [P(CH3)4]+ ranged over 1.667 to -1.060 the colour spectrum the snap-shots were taken was set to 2.000 to -2.000 to encompass both compounds’ charge ranges. The symmetry of both cations is Td.
distributions as EN effects decrease with distance and they are two bonds away from the nitrogen and phosphorus centres. The charge on the hydrogen atoms is 0.269 and 0.298 in [N(CH3)4]+ and [P(CH3)4]+ respectively. The central nitrogen ion has a charge of -0.295, and the carbon atoms -0.484 each. The central phosphorus ion has a charge of 1.667, with the carbon atoms being -1.060 each. This charge distribution is attributed to the difference in electronegativities: nitrogen is more electronegative than carbon, so it is negatively charged and the carbon atoms are expected to be less negatively charged. Whereas, phosphorus is more electropositive than carbon, so it is positively charged and the carbons are negatively charged. The hydrogens have similar charge distributions as EN effects decrease with distance and they are two bonds away from the nitrogen and phosphorus centres.
On that note the actual charge observed on nitrogen in [N(CH3)4]+ is -0.295, and the charge observed on carbon atoms is -0.484 , which is more negative opposite of what is theorised. The reason for this is nature of nitrogen and the small difference in EN of the atoms. Carbon has an EN of 2.55 and nitrogen has an EN of 3.04. Compared to the EN of oxygen which is 3.44 the difference in EN values of carbon and nitrogen is half therefore, nitrogen could be expected to have a less negative charge as it also shares its electron density more flexibly than carbon. In fact nitrogen shares its electron density with the methyl groups to form the [N(CH3)4]+ cation.[3]
Here you needed to consider more the comparative C charge between the molecules, it is more postive (less negative) in [N(CH3)4]+ compared to in [P(CH3)4]+ which is the effect of the N-C relative electronegativities. Smf115 (talk) 21:21, 20 May 2019 (BST)
Nitrogen and Phosphorus are Group 5 elements found in the 2nd an 3rd rows respectively. Therefore, the orbital overlap between the nitrogen 2s, 2p orbitals and the methyl orbital fragments (carbon – 2s, 2p) will be more efficient, than the phosphorus 3s, 3p orbital overlap with the methyl fragments. As orbital efficiency decreases with increasing element size the diffuse phosphorus orbital overlap with the methyl fragments is less efficient. Consequently, the symmetry of the phosphorus cation is expected to be uneven compared to the nitrogen cation; in fact the charge distribution of [P(CH3)4]+ is over a larger range confirming this. Furthermore, nitrogen is small and highly EN so the central nitrogen and the methyl groups are expected to be closer together and the nitrogen cation more symmetric due to increased overlap. Phosphorus on the other hand is and forms longer bonds to the methyl groups so it’s expected to be less symmetric due to decreased overlap. Indeed the bond lengths of the [N(CH3)4]+ C – N and [P(CH3)4]+ P – N were measured as 1.509 Å and 1.816 Å respectively.
As a last note based on the prior analysis of the charge distribution the C – P bond is assumed to have a greater ionic character than the C – N bond which is expected to have a greater polar covalent character.
Correct charges calculated, good attention to the colour range used across both molecules and clearly presented charges. Your initial discussion of the electronegativities and comparison of the molecules is really good and clear. However, while some of the later ideas are interesting they are wrong in several places. For example, both molecules correctly have the same Td symmetry, but then you go on to say that [N(CH3)4]+ is more symmetric than [P(CH3)4]+? Smf115 (talk) 21:21, 20 May 2019 (BST)
The formal charge represents the overall charge of the molecule assuming the charge is spread equally among the atoms. In the traditional sense, the ionic plus is placed on the central atom to represent the entire molecule being formally positive, but converging on the nitrogen. The reason [NR4]+ species are depicted with a formal positive charge on the nitrogen atom is due to electron sharing model in covalent bonding. Formal charge is allocated such as electrons are distinct particles and shared equally in a single bond. The EN and wave – particle duality effects are neglected in the traditional model for depicting molecules. Therefore, as nitrogen bonds to three R groups to fulfil its octet, the fourth R bond forms by electron density sharing between the nitrogen and the R group carbon. Hence, the positive formal charge should be located on the R groups in order to reflect the actual bonding within the molecule more accurately.
You should have considered formal electron counting here when thinking about how the traditional picture arises. Also looking at your NBO charges, where does the positive charge actually sit? Smf115 (talk) 21:21, 20 May 2019 (BST)
MO Analysis
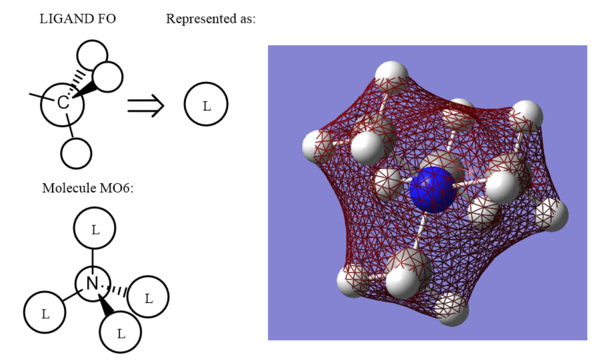
The energy of the MO is -1.19645 au. This MO has a1 summetry and consists of fully bonding CH3 orbital fragments (Carbon 2s orbital & Hydrogen 1s orbitals) and the Nitrogen 2s orbital. There is strong s-s bonding through the bonds and weak s-s bonding through space the MO is therefore, fully bonding.
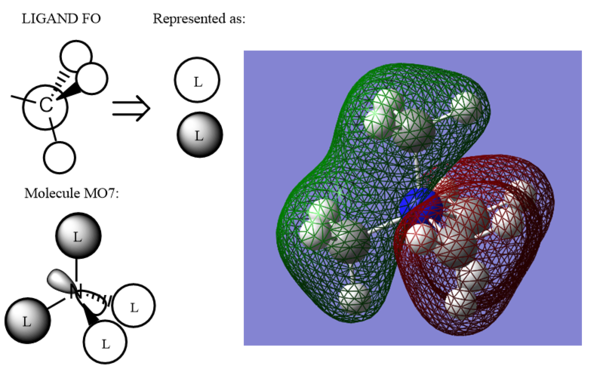
The energy of the MO is -0.92554 au. This MO consists of strongly bonding CH3 orbital fragments (Carbon 2s orbital & Hydrogen 1s orbitals) and the Nitrogen 2p orbital. There is medium s-p and s-s bonding through the bonds, and weak s-p and s-s anti bonding through space; the MO has therefore, mostly bonding character.
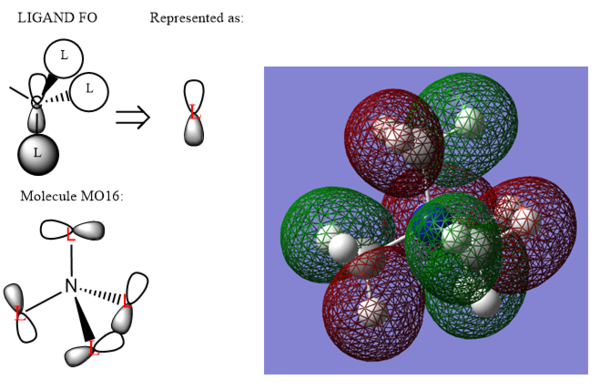
The energy of the MO is -1.58031 au. This MO consists of medium bonding CH3 orbital fragments (Carbon 2p orbital & Hydrogen 1s orbitals). There is weak s-p anti bonding through space and weak p-p anti bonding through space. The MO has therefore weakly bonding character and is a non-bonding MO.
Clear presentation, correct FOs and LCAOs and great evaluation of the overall character of the MO. The only improvement would be to see a bit more of a range of MOs chosen as 6 and 7 are quite low in terms of complexity. Otherwise great and very clear! Smf115 (talk) 22:30, 21 May 2019 (BST)
Excellent report with a very well done section 1. Smf115 (talk) 22:30, 21 May 2019 (BST)