Rep:Mod:MA8113
Transition States
In this exercise Diels-Alder reactions would be experimented computationally using the Gaussian software. This exercise involves determining the transition states and also analysing the HOMO/ LUMO molecular orbitals involved.
What is a transition state and a minimum?
A transition state is the highest energy structure that lies between the reactants and products.
Energy minimum is the minimum energy point along a reaction coordinate.
Nf710 (talk) 20:10, 7 February 2017 (UTC) This is a poor explanation, you need to talk about second derivatives wrt to the PES
1. Exercise 1
The 1,3 butadiene and ethylene were optimized at the PM6 level and the bond distance frozen at 2.2 angstroms using the redundant coordinate editor. Further calculations of optimization to a minimum were carried out to the output file. Optimization to a TS bermy and frequency analysis of the log file at the PM6 level, with the force constant being calculated once produced a transition state output file. Further analysis of the transition state with the IRC method resulted in the output data as shown below in this section.
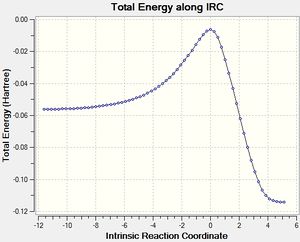
The IRC calculated results are shown below:
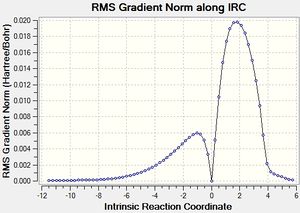 |
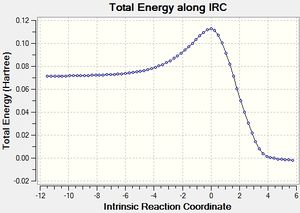 |
HOMO and LUMO Molecular Orbitals
LUMO |
HOMO | |
|---|---|---|
1,3 Butadiene(Diene) |
||
Ethylene(Dienophile) |
||
Transition State |
For the Diels-Alder reaction to take place the diene must be in the cis conformation.The molecular orbital diagram of the Diels-Alder reaction with 1,3 butadiene and ethene is shown below.
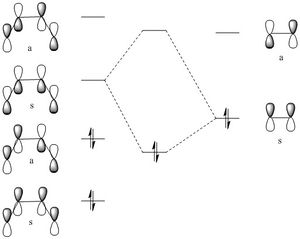
This Diels-Alder reaction is thermally allowed in accordance with the Woodward-Hoffmann rule, see the analysis of the frontier orbitals in figure 3.
 |
 |
|---|
The bonds lengths of the reactants:
The 4 C-C length of the 1,3 butadiene reactant is 4.2 angstrongs, see figure 2 (C1-C6).
The C-C length of ethylene is 1.4 angstrongs, see figure 2 (C11-C12)
The 6 C-C length of the transition state is 5.6 angstrong
The C-C bond lengths of the product is 8.96 angstrong
The van der Waals radius of a carbon atom is 1.70 angstrong this is larger than 1.42 angstrongs of a partially formed C-C bond.[1]
Orbital overlap:
Symmetric- Asymmetric would give a zero integral
Symmetric- Symmetric would give a non-zero integral
Asymmetric- Asymmetric would give a zero integral
Nf710 (talk) 20:16, 7 February 2017 (UTC) This section is very brief and you have missed a few things such as vibrations.
Exercise 2:
Endo product
The reactants were optimized at a minimum PM6 level and the transition state was obtained using frequency and optimization method at a PM6 level. Analysis of the vibration result showed a value of -960.04 cm-1. The HOMO and LUMO of the endo transition state is shown below:
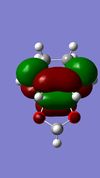 |
 |
(These images need context. How were they formed? What's the significance of their symmetry? Tam10 (talk) 11:36, 7 February 2017 (UTC))
Reaction coordinate Diagram:
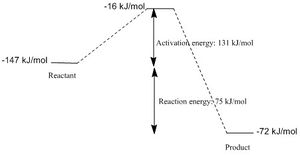 |
The activation energy of the reaction is 131 kJ/mol
and the reaction energy is 75 kJ/mol. |
|---|
(The energy level values on the diagram imply a positive reaction energy Tam10 (talk) 11:36, 7 February 2017 (UTC))
This reaction is a normal electron demand Diels-Alder reaction.
The endo product is the thermodynamic product. The IRC for this reaction is given below:
Exo Product
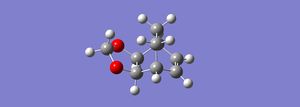
Optimizing the reaction at the PM6 level and at a set distance of 2.2 angstrong and further optimization and frequency calculation using the PM6 level method, with the bonds frozen obtain a transition state with a negative frequency of -958.64. The HOMO and LUMO of the Exo product are given below.
 |
 |
|---|
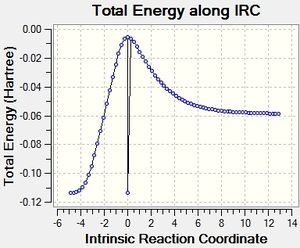
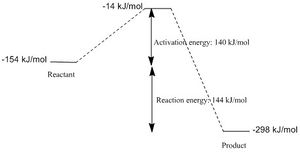
The exo product is the kinetic product and the reaction is an inverse demand Diels-Alder reaction. IRC analysis obtained by selecting the force constant as always and following the path in both direction at the PM6 level, obtain results as shown below. Also shown is the reaction coordinate energy diagram with the activation energy and the overall reaction energy.
 |
Reaction coordinate:
The activation energy is 140 kJ/mol and the reaction energy is 144 kJ/mol. |
|---|
Nf710 (talk) 20:24, 7 February 2017 (UTC) There is a large discrepancy between your energies, they should be pretty similar. Also your orbitals are unclear they should be the same symmetry (endo, exo), and your have come to the incorrect conclusions about what is the thermo and kenetic product. Sorry about that. You also didnt show ant SOO.
Exercise 3: Diels-Alder vs Cheletropic reaction
(Section is incomplete and missing the endo DA reaction Tam10 (talk) 11:36, 7 February 2017 (UTC))
1. Diels-Alder
 |
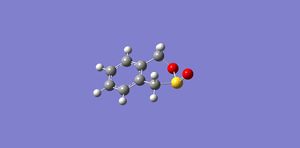 |
|---|
The IRC calculation using the PM6 method with calculation of the force constant once in both directions the result is outlined below:
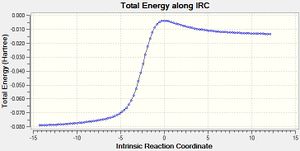 |
 |
|---|
The activation energy of this reaction 25 kJ/mol and the overall change in energy is 172 kJ/mol
The reaction coordinate is given below:
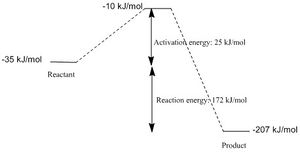
2. Cheletropic
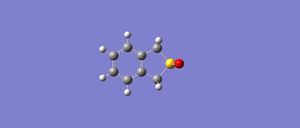 |
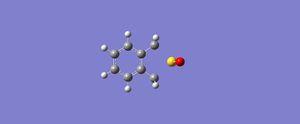 |
|---|
To conclude, I have been able to optimise and determine the transition states and obtain successfully the HOMO/ LUMO molecular orbitals involved of the various Diels-Alder reactions using the variety of methods listed in the tutorial exercise.
- ↑ S.S. Batsanov, Inorg. Mater., 2001, 37 (9), 871-885.