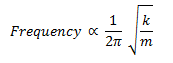Rep:Mod:Linstead
Optimisation
BH3 First Optimisation
BH3
Summary
| Title | Result |
|---|---|
| File Name | BH3 |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 3-21G |
| Final Energy (au) | -26.46226338 |
| Gradient | 0.00020672 |
| Dipole Moment | 0.00 |
| Point Group | D3H |
| Duration of Calculation | 10 seconds |
Convergence Report
Item Value Threshold Converged?
Maximum Force 0.000413 0.000450 YES
RMS Force 0.000271 0.000300 YES
Maximum Displacement 0.001610 0.001800 YES
RMS Displacement 0.001054 0.001200 YES
Predicted change in Energy=-1.071764D-06
Optimization completed.
-- Stationary point found.
BH3 Second Optimisation
BH3
Summary
| Title | Result |
|---|---|
| File Name | BH3_II |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy (au) | -26.61532363 |
| Gradient | 0.00000296 |
| Dipole Moment | 0.00 |
| Point Group | D3H |
| Duration of Calculation | 8 seconds |
Convergence Report
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000023 0.001800 YES
RMS Displacement 0.000015 0.001200 YES
Predicted change in Energy=-2.008855D-10
Optimization completed.
-- Stationary point found.
TlBr3
DSpace Report
Summary
| Title | Result |
|---|---|
| File Name | TlBr3_LOG_ALF |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | LANL2DZ |
| Final Energy (au) | -91.21812851 |
| Gradient | 0.00000090 |
| Dipole Moment | 0.00 |
| Point Group | D3H |
| Bond Length (Å) | 2.65095 |
| Bond Angle (deg) | 120 |
| Duration of Calculation | 19.6 seconds |
Convergence Report
Item Value Threshold Converged?
Maximum Force 0.000002 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000022 0.001800 YES
RMS Displacement 0.000014 0.001200 YES
Predicted change in Energy=-6.084023D-11
Optimization completed.
-- Stationary point found.
Literature bond lengths put the Tl-Br bond at 2.5122 Å [1], which means the value given by this model, 2.65095 Å is reasonable. The variation between my modeled values and the experimental values arise due to the limits of using only a few wave functions and small basis sets.
Optimisation BBr3
DSpace Report
http://dx.doi.org/10042/23338
Summary
| Title | Result |
|---|---|
| File Name | BBr3_LOG_ALF |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | Gen |
| Final Energy (au) | -64.43645253 |
| Gradient | 0.00012323 |
| Dipole Moment | 0.00 |
| Point Group | D3H |
| Bond Length (Å) | 1.933 |
| Bond Angle (deg) | 120 |
| Duration of Calculation | 31.5 seconds |
Convergence Report
Item Value Threshold Converged?
Maximum Force 0.000246 0.000450 YES
RMS Force 0.000161 0.000300 YES
Maximum Displacement 0.001151 0.001800 YES
RMS Displacement 0.000754 0.001200 YES
Predicted change in Energy=-4.716315D-07
Optimization completed.
-- Stationary point found.
Frequency Analysis
BH3
Vibrational Frequencies Summary
Low frequencies --- -0.9432 -0.8611 -0.0054 5.7455 11.7246 11.7625 Low frequencies --- 1162.9963 1213.1826 1213.1853
IR Spectrum
A computed IR spectrum of the calculated molecule is included:

There appear to be only 3 peaks rather than 6 because the totally symmetric stretch does not give a change in dipole, and therefore does not appear. The degenerate peaks appear at the same energy, and thus are not distinguishable. This means you only see 3 distinguishable peaks.
TlBr3
DSpace Report
Vibrational Frequencies Summary
Low frequencies --- -3.4213 -0.0026 -0.0004 0.0015 3.9367 3.9367 Low frequencies --- 46.4289 46.4292 52.1449
IR Spectrum
A computed IR spectrum of the calculated molecule is included:

Comparison of IR frequencies in the isostructural compounds TlBr3 and BH3
| # | TlBr3 | BH3 |
|---|---|---|
| 1 | 46 E' | 1163 A2" |
| 2 | 46 E' | 1213 E' |
| 3 | 52 A2" | 1213 E' |
| 4 | 165 A1' | 2582 A'1 |
| 5 | 211 E' | 2715 E' |
| 6 | 211 E' | 2715 E' |
The large difference in the frequency values between BH3 and TlBr3 indicates the large difference in mass and also the differing in strength of the bonds within the molecule.
The equation that relates mass of the system and the frequency is given below:
There is also the fact that the force constant will be different in each system, because boron and hydrogen are smaller than thallium and bromide, the bonds will be shorter and stiffer, giving a larger force constant. This will also serve to increase the frequency of the BH3 stretches in comparison to the TlBr3.
There has been a reorganisation of stretching modes, in that the A2" is the lowest energy mode for the BH3, but the A2" comes after the two E' modes in the TlBr3.
The spectra are similar in that there are two peaks much smaller than the third, due to degenerate stretches.
Optimisation and Frequenc Analysis Methods
The molecules must be optimised using exactly the same basis sets and methods, so that the assumptions used in each case are the same. If you were to use different basis sets for the same molecule it would give us different minima and differing stretching frequencies.
The frequency analysis allows us not only to determine that we have found a minima, by minimising the low frequencies, but also allow us to see stretching modes that would otherwise not appear on a spectra, because of being totally symmetric, not changing the dipole moment.
The low frequencies that are shown in the .log file are the "-6" in the 3N-6 stretching modes.
Comparison of Bond Lengths in BH3, BBr3 and TlBr3
| Molecule | Bond length / Å |
| BH3 | 1.19 |
| TlBr3 | 2.65 |
| BBr3 | 1.93 |
The BH3 is the smallest molecule, with both the atoms involved being very small. This results in a far smaller bond length than even the BBr3. The BBr3 has three bromide atoms, each of which is far far greater in size than a hydrogen. The difference in bond lengths is also down to the fact that the valence shell for the bromide is far more diffuse than that of the hydrogen, and that there are three lone pairs on the bromide atom, each taking up space. The same logic applies to the TlBr3, where the thallium is both larger and has a more diffuse valence shell than the boron.
The atoms themselves have differing covalent radii, and when you look at these, the trend is the same.
| Molecule | Atomic Radius [2]/ Å |
| H | 0.32 |
| B | 0.84 |
| Br | 1.17 |
| Tl | 1.44 |
Overall, the steric bulk of the constituent atoms along with their electronegativity makes up for the difference in bond lengths.
What is a bond?
On Gaussview, a bond is determined by length. Whether the distance between two atoms is within a set "bond condition." This leads to some confusion when Gaussview appears to show no bond between two atoms that are in fact bonded. This is because the bond length falls outside what Gaussview calls a bond. It is in fact the overlap of orbitals that make up a bond, and the IUPAC definition of a bond is "A region of relatively high electron density between nuclei which arises at least partly from sharing of electrons and gives rise to an attractive force and characteristic internuclear distance." [3] Using this as our guide, we can see that a bond should be solved using quantum mechanics to find the electron density between two atoms. This means that a bond does not suddenly come into being, as Gaussview appears to show, but increases its bonding character as the atoms approach from infinitely far apart.
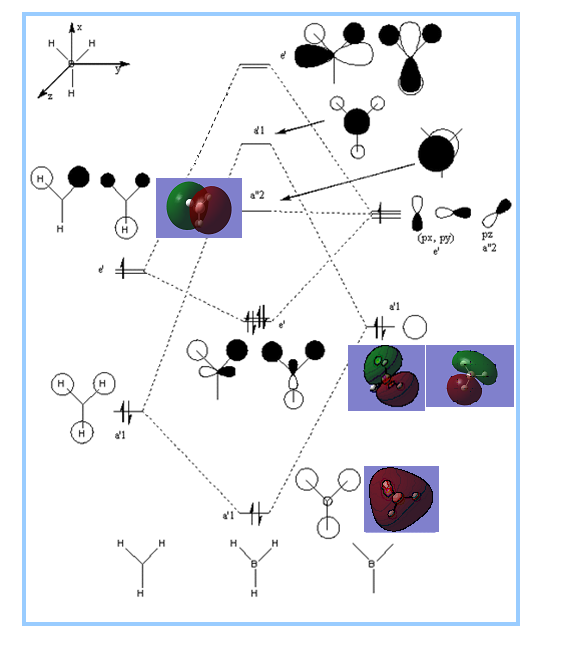
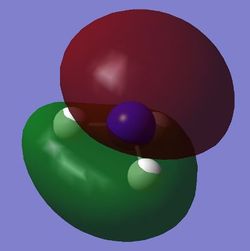
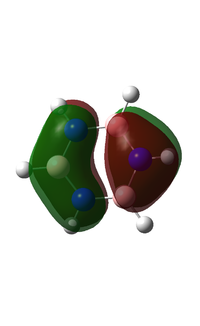
Molecular orbitals
BH3
DSpace Report
MO Diagram
NH3
Optimisation of NH3 molecule:
| Title | Result |
|---|---|
| File Name | NH3_ALF_II |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RHF |
| Basis Set | 6-31G(d,p) |
| Final Energy (au) | -56.19554454 |
| Gradient | 0.00010676 |
| Dipole Moment (Debye) | 1.8 |
| Point Group | C1 |
| Bond Length (Å) | 1.000 |
| Bond Angle (deg) | 107.6 |
| Duration of Calculation | 11 seconds |
Item Value Threshold Converged?
Maximum Force 0.000200 0.000450 YES
RMS Force 0.000124 0.000300 YES
Maximum Displacement 0.000478 0.001800 YES
RMS Displacement 0.000328 0.001200 YES
Predicted change in Energy=-1.231704D-07
Optimization completed.
-- Stationary point found.
Frequency Analysis:
Low frequencies --- -52.9939 -51.3795 -49.1847 -0.0016 0.0013 0.0014 Low frequencies --- 1141.4485 1810.4326 1810.6379
Re-Optimisation and Frequency Analysis:
| Title | Result |
|---|---|
| File Name | NH3_ALF_II_FREQA |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RHF |
| Basis Set | 6-31G(d,p) |
| Final Energy (au) | -56.19554465 |
| Gradient | 0.00000256 |
| Dipole Moment (Debye) | 1.8 |
| Point Group | C1 |
| Bond Length (Å) | 1.000 |
| Bond Angle (deg) | 107.6 |
| Duration of Calculation | 3 seconds |
Low frequencies --- -6.0649 -5.1190 -3.1423 -0.0014 0.0012 0.0018 Low frequencies --- 1142.1708 1810.8159 1810.8163
Further Re-optimisation
The previous optimisations were carried out with the hartree fock energy, which was incorrect. A further optimisation was run with the correct energy set.
File:NH3 ALF II FREQA FINAL.LOG
| Title | Result |
|---|---|
| File Name | NH3_ALF_II_FREQA_Final |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy (au) | -56.55776872 |
| Gradient | 0.00000326 |
| Dipole Moment (Debye) | 1.8 |
| Point Group | C1 |
| Bond Length (Å) | 1.02 |
| Bond Angle (deg) | 105.7 |
| Duration of Calculation | 13 seconds |
Low frequencies --- -3.7752 0.0011 0.0013 0.0013 5.2394 7.0728 Low frequencies --- 1089.3784 1693.9290 1693.9383
MO Analysis
| Title | Result |
|---|---|
| File Name | NH3_ALF_II_MO |
| File Type | .log |
| Calculation Type | SP |
| Calculation Method | RHF |
| Basis Set | 6-31G(d,p) |
| Final Energy (au) | -56.19554465 |
| Gradient | 0.00000256 |
| Dipole Moment (Debye) | 1.8 |
| Point Group | C1 |
| Bond Length (Å) | 1.000 |
| Bond Angle (deg) | 107.6 |
| Duration of Calculation | 3 seconds |
The pictures of the MOs are displayed below:
NBO Analysis
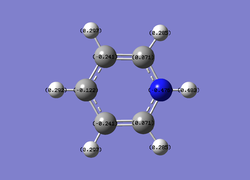
The specific charge for the Nitrogen is -1.131, and each Hydrogen is +0.377, so the molecule is neutrally charged.
NH3BH3
| Title | Result |
|---|---|
| File Name | NH3BH3_ALF |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy (au) | -83.22468957 |
| Gradient | 0.00005842 |
| Dipole Moment (Debye) | 5.565 |
| Point Group | C1 |
| Bond Length N-B (Å) | 1.668 |
| Lit. [4] Bond Length N-B (Å) | 1.601 |
| Duration of Calculation | 55 seconds |
| Title | Result |
|---|---|
| File Name | NH3BH3_ALF_FREQ |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy (au) | -83.22468990 |
| Gradient | 0.00005729 |
| Dipole Moment (Debye) | 5.565 |
| Point Group | C1 |
| Bond Length N-B (Å) | 1.668 |
| Lit. [4] Bond Length N-B (Å) | 1.601 |
| Duration of Calculation | 39 seconds |
| Title | Result |
|---|---|
| File Name | NH3BH3_ALF_FREQ |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy (au) | -83.22468904 |
| Gradient | 0.00000124 |
| Dipole Moment (Debye) | 5.565 |
| Point Group | C1 |
| Bond Length N-B (Å) | 1.668 |
| Lit. [4] Bond Length N-B (Å) | 1.601 |
| Duration of Calculation | 1 minute 25 seconds |
Instead of looking at the total energy of the molecule, I have decided to look at the free energy, including the entropic term. This gives you a lower value, since you are of course going from two molecules to one, and losing entropy. This takes up energy and thus the free energy of the bond is lower than the ~135kJ/mol that is expected.
Free Energy: NH3BH3 @ 298K: -83.178118 Free Energy: NH3 @ 298K: -56.542403 Free Energy: BH3 @ 298K: -26.606441 Free Energy of reaction: -0.029274 au Free Energy of reaction kJ/mol: -76.86 Free Energy of dissociation @ 298K: 80kJ/mol
Investigating Aromaticity
Benzene
Benzene is the standard with which all aromatic molecules are compared. The molecular orbitals however are not as simple as first imagined.
Optimisation and Frequency Analysis
| Title | Result |
|---|---|
| File Name | Benzene_ALF_1 |
| File Type | .log |
| Calculation Type | Freq |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy (au) | -232.25820551 |
| Gradient | 0.00009550 |
| Dipole Moment (Debye) | 0 |
| Point Group | C1 |
| Bond Length C-C (Å) | 1.396 |
| Bond Length C-H (Å) | 1.086 |
| Bond Angle (deg) | 120 |
| Duration of Calculation | 4minutes 35 seconds |
Item Value Threshold Converged?
Maximum Force 0.000212 0.000450 YES
RMS Force 0.000085 0.000300 YES
Maximum Displacement 0.000991 0.001800 YES
RMS Displacement 0.000315 0.001200 YES
Predicted change in Energy=-5.157454D-07
Optimization completed.
-- Stationary point found.
Low frequencies --- -17.2824 -14.5873 -9.6634 -0.0008 -0.0004 0.0003 Low frequencies --- 413.7969 414.4697 620.8546
MO Analysis
Boratabenzene
Optimisation and Frequency Analysis
| Title | Result |
|---|---|
| File Name | Pyri_ALF_1 |
| File Type | .log |
| Calculation Type | Freq |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy (au) | -219.02052984 |
| Gradient | 0.00015815 |
| Dipole Moment (Debye) | 2.85 |
| Point Group | C1 |
| Bond Length C-C (Å) | 1.40 |
| Bond Length C-B (Å) | 1.514 |
| Average Bond Angle C-C-C (deg) | 121 |
| Bond Angle C-B-C (deg) | 115.0 |
| Duration of Calculation | 3minutes 16 seconds |
Item Value Threshold Converged?
Maximum Force 0.000160 0.000450 YES
RMS Force 0.000069 0.000300 YES
Maximum Displacement 0.000861 0.001800 YES
RMS Displacement 0.000321 0.001200 YES
Predicted change in Energy=-7.184243D-07
Optimization completed.
-- Stationary point found.
Low frequencies --- -13.1331 -0.0005 -0.0001 0.0006 15.0724 18.1749 Low frequencies --- 371.3449 404.2342 565.2519
MO Analysis
Further analysis will follow when comparing to other aromatic systems.
Pyridinium
Optimisation and Frequency Analysis
| Title | Result |
|---|---|
| File Name | Pyri_ALF_1 |
| File Type | .log |
| Calculation Type | Freq |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy (au) | -248.66807396 |
| Gradient | 0.00003904 |
| Dipole Moment (Debye) | 1.87 |
| Point Group | C1 |
| Bond Length C-C (Å) | 1.398 |
| Bond Length C-N (Å) | 1.352 |
| Bond Angle C-C-C (deg) | 119.3 |
| Bond Angle C-N-C (deg) | 123.0 |
| Duration of Calculation | 3minutes 16 seconds |
A jmol file can be found
Item Value Threshold Converged?
Maximum Force 0.000064 0.000450 YES
RMS Force 0.000023 0.000300 YES
Maximum Displacement 0.000836 0.001800 YES
RMS Displacement 0.000186 0.001200 YES
Predicted change in Energy=-7.441419D-08
Optimization completed.
-- Stationary point found.
Low frequencies --- -7.1994 -0.0007 0.0003 0.0006 17.3377 18.5318 Low frequencies --- 392.4552 404.0618 620.4715
MO Analysis
Further analysis will follow when comparing to other aromatic systems.
Borazine
Optimisation and Frequency Analysis
| Title | Result |
|---|---|
| File Name | Borazine_ALF_I |
| File Type | .log |
| Calculation Type | Freq |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy (au) | -242.68459771 |
| Gradient | 0.00002102 |
| Dipole Moment (Debye) | 0 |
| Point Group | C1 |
| Bond Length B-N(Å) | 1.431 |
| Bond Length B-H (Å) | 1.195 |
| Bond Length N-H (Å) | 1.010 |
| Bond Angle B-N-B (deg) | 122.9 |
| Bond Angle N-B-N (deg) | 117.1 |
| Duration of Calculation | 3minutes 15 seconds |
Item Value Threshold Converged?
Maximum Force 0.000072 0.000450 YES
RMS Force 0.000018 0.000300 YES
Maximum Displacement 0.000416 0.001800 YES
RMS Displacement 0.000156 0.001200 YES
Predicted change in Energy=-4.391525D-08
Optimization completed.
-- Stationary point found.
Low frequencies --- -11.2213 -0.0012 -0.0007 -0.0006 8.2077 9.4691 Low frequencies --- 288.6131 290.4743 404.3232
MO Analysis
Further analysis will follow when comparing to other aromatic systems.
Aromatic Comparison
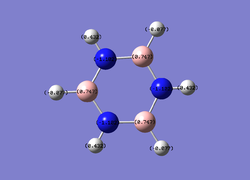
NBO Charge Distribution
MO Comparison
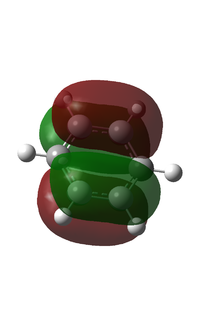
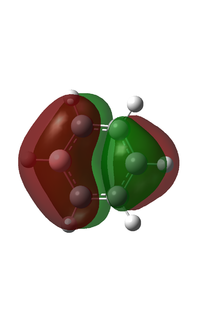
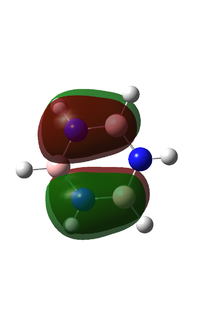
I have chosen to compare the fully p based MOs.
MO 17
MO 20
MO 21
Analysis
The most important point that is visible in all the MOs, is that the boron, being less electronegative than the carbon, has less electron density on it. This logic applies to the carbon as compared to the more electronegative nitrogen. The energies of the orbitals also became increasingly negative along the same scale, boron < carbon < nitrogen. Having a more electronegative heteroatom in the system lowers the energy of that system. When both nitrogen and boron atoms were present, in the borazine, the effects of each were vaguely cancelled out, with the energies being similar, although slightly more negative in all cases, to the energy of benzene. We can assume that the lowering in energy because of the inclusion of the electronegative element is due to its more readily accepting electrons. This would lower bonding orbitals and raise antibonding orbitals.
Generally, the order of MOs was unchanged from molecule to molecule, especially in the frontier region. The degeneracy of the orbitals was similar in the four, but with some small differences. Benzene is the most degenerate of the four, and is also the most symmetrical, whereas boratabenzene and pyridinium share degeneracy and symmetry. This leads us to the conclusion that symmetry and degeneracy are related.
Probably the most interesting thing to be gained from this exercise was that using a small basis set and a simple method, an accurate picture of the orbitals was derived. This shows that using quantum mechanics you can solve for most/any molecule to give a good approximation.