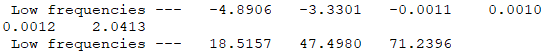Rep:Mod:Lazarus Comp
BH3
B3LYP/6-31G level
Summary
Frequencies
Structure
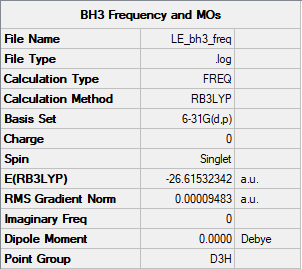
BH3 |
Ng611 (talk) 15:26, 13 May 2019 (BST) Where is your .log file?
IR Spectrum
| wavenumber (cm-1) | Intensity (arbitrary units) | symmetry | IR active? | type |
|---|---|---|---|---|
| 1163.72 | 92 | A2" | yes | out-of-plane bend |
| 1213.67 | 14 | E' | slightly | bend |
| 1213.67 | 14 | E' | slightly | bend |
| 2579.74 | 0 | A1' | no | symmetric stretch |
| 2712.67 | 126 | E' | yes | asymmetric stretch |
| 2712.67 | 126 | E' | yes | asymmetric stretch |
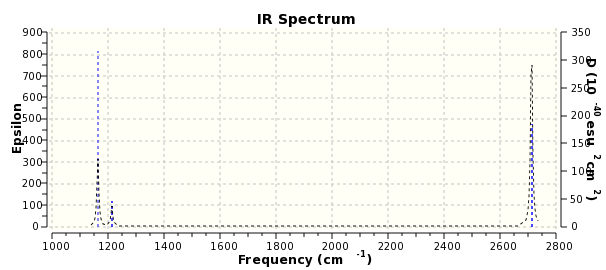
Despite having 6 vibrations, the spectrum only shows 3 peaks. The peak at 1213 cm-1 is representative to 2 peaks which have the same energy. The same applies for the peaks at 2712 cm-1. The final vibration which is not present on the spectrum is at 2579 cm-1 and this is due to the vibration being symmetrical and so it has no effect on the dipole moment.
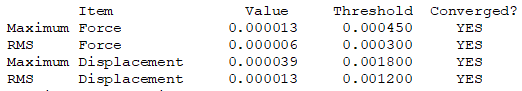
Convergence
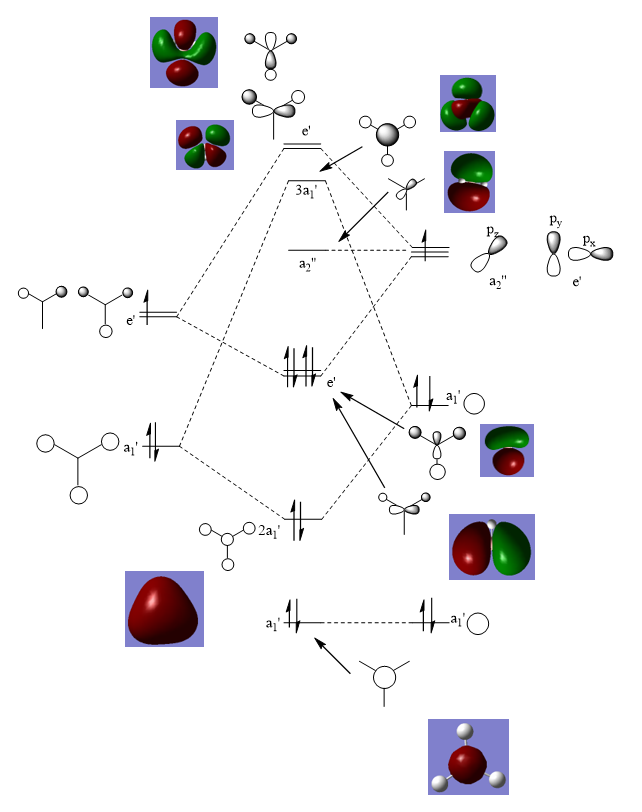
Molecular Orbitals
The real MOs are slightly different to the LCAO MO. This is because the LCAO MOs are able to show the interactions between orbitals without optimisation. The optimised real MOs show a more uniform combinations and take into account certain factors. However, the LCAO MOs are very good at providing a starting point from which real MOs can expand upon and form more accurate representations of bonding in atoms.
Ng611 (talk) 15:24, 13 May 2019 (BST) You're correct that differences exist, but your explanation is too vague. What do you mean by "a more uniform combinations?"
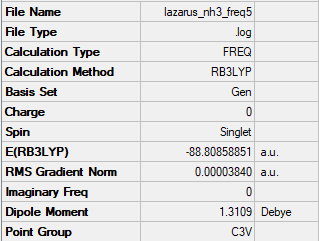
NH3
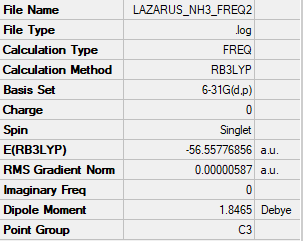
Summary
Frequencies
Convergence
NH3 |
H3BNH3
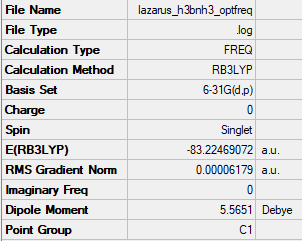
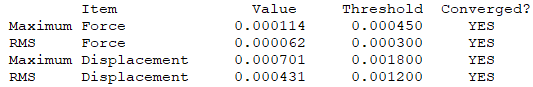
Summary
Frequencies
Convergence
H3BNH3 |
Energy calculations
E(NH3)= -56.55776856 a.u.
E(BH3)= -26.61532342 a.u.
E(H3BNH3)= -83.22469072 a.u.
ΔE= E(NH3BH3)-[E(NH3)+E(BH3)] ΔE= -0.05159874 au = -135.4466925 kJ/mol
Ng611 (talk) 15:28, 13 May 2019 (BST) Good calculation! However, you've reported your answer to far too many decimal places.
The B-N dative bond is a fairly weak bond especially when compared to an iso-electronic C-C bond which requires 346 kJ/mol to break, which is approximately 3 times stronger per mole.
Ng611 (talk) 15:28, 13 May 2019 (BST) Good comparison, but you need to cite the source you obtained your bond enthalpy from.
NI3
Summary
Ng611 (talk) 15:32, 13 May 2019 (BST) You've missed out your .log file again. Without this, it's impossible to check your pseudopotential input.
Convergence
Frequencies
NI3 |
2.184 Å: optimised bond distance
Lewis Acids and Bases
Isomers of Al2Cl4Br2
Symmetry of isomers
Ng611 (talk) 15:38, 13 May 2019 (BST) It looks like you used a pseudopotential input that includes a pseudopotential for Cl as well. This has led to incorrect final answers. Remember that pseudopotentials are only used for heavier atoms (e.g.: bromine) that have a significant number of core electrons.
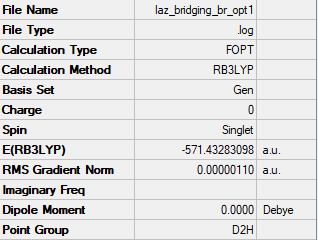
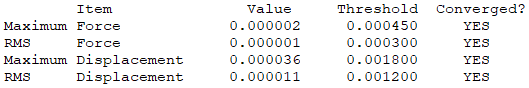
Isomer 1: D2h
The frequency file is linked here
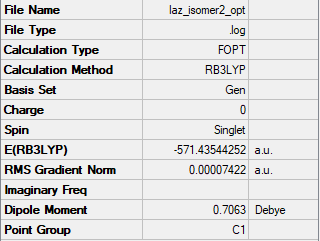
Isomer 2: C1
The frequency file is linked here
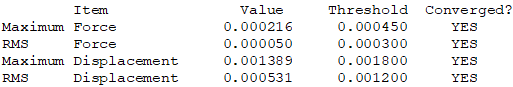
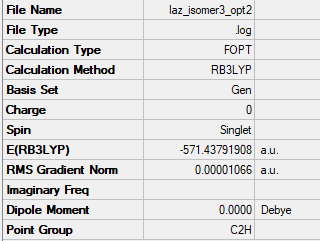
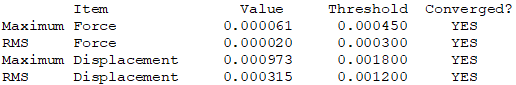
Isomer 3: C2h
The frequency file is linked here
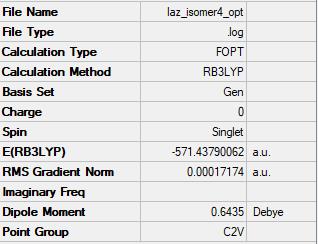
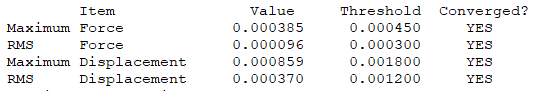
Isomer 4: C2v
The frequency file is linked here
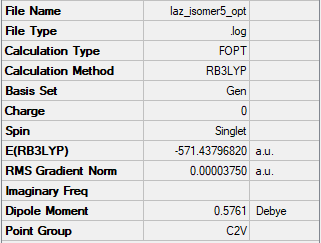
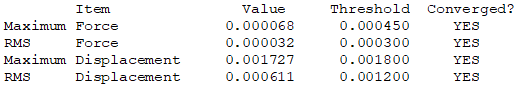
Isomer 5: C2v
The frequency file is linked here
Energies in order from least to most stable:
Isomer 1: -1500297.0120246 kJ/mol
Isomer 2: -1500303.8686233 kJ/mol
Isomer 4: -1500310.3223654 kJ/mol
Isomer 3: -1500310.3708321 kJ/mol
Isomer 5: -1500310.4997967 kJ/mol
Conformers where Br is either one or two of the bridging ions are approximately 10 kJ/mol weaker than those where Cl ions are both bridging atoms. This is due to the weaker overlap between aluminium and bromine orbitals in comparison to the aluminium and chlorine orbitals. This weaker overlap means less energy is needed to break the bonds.
Dissociation energy
The monomer, AlCl2Br, has a dissociation energy of -750113.30356815 kJ/mol and so the ΔE= 2(-750113.30356815) - (-1500310.3708321) = 83.7636958 kJ/mol. These results show that the dimer is significantly more stable than two monomers and means that the dimer is more likely to form. Given that the difference in energy is so large, the 3c-2e bonding is clearly more favourable for this structure as opposed to the 2c-2e bonding seen in the monomers.
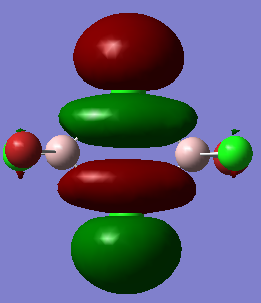
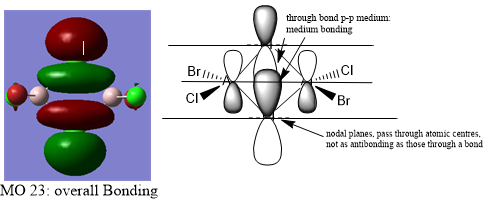
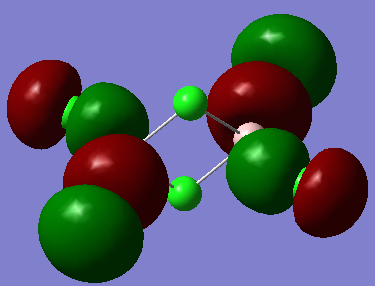
MO visualisation
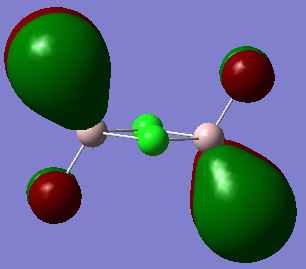
MO 23:
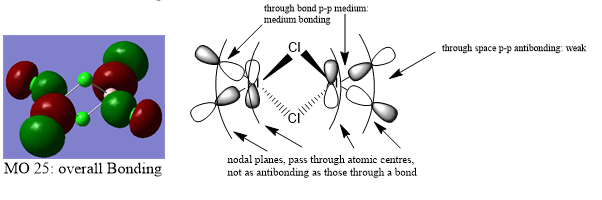
MO 25:
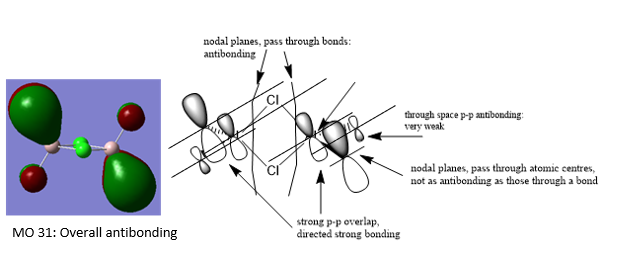
MO 31:
It is very clear through the combination of the visualisation of MO's and the dissociation energy calculation, that the br ion bridging significantly destabilises the compound due to its poor orbital overlap. Chlorine ions are significantly better placed to be the bridging ions since they are a period above Br ions and so have much better overlap of orbitals.
Ng611 (talk) 15:55, 13 May 2019 (BST) Your LCAOs don't seem incorrect, but I think generally you need to improve your labelled diagrams. For MO31 in particular, it was very difficult for me to understand the interactions.