Rep:Mod:LT611
Experiment 1C: Part 1
Conformational analysis using for Molecular Mechanics
The Hydrogenation of Cyclopentadiene Dimer
Theoretically, the dimerization of cyclopentadiene via a Diels-Alder reaction should yield both the exo and endo product, however experimentally, only the endo (dimer 2) is observed. 2D illustrations of both of the dimers can be seen in figure 1. To establish whether the cyclodimerisation of cyclopentadiene is thermodynamically or kinetically controlled, the relative stabilities of dimers 1 and 2 were compared using ChemBio3D software under a MM2 force field. It can be seen from the results, shown in table 1 that dimer 2 has a total energy of 33.9976 kcal/mol whereas dimer 1 only has a total energy of 31.8764 kcal/mol. Total energy has been calculated as the sum of all of the other energies in the table. The difference between the endo and exo products lies in the torsional strain where the exo product has an energy of approximately 2.1 kcal/mol less than the endo product. Thus if the reaction was under thermodynamic control, we would anticipate the product to be exclusively 1. Hence, it can be concluded that the dimerisation of cyclopentadiene must be under kinetic control as the major product of the reaction has the highest energy. This is caused by the resonance form of the transition state, which is a zwitterion, being in a favourable orientation for the endo dimer as the positive and negative charges are overlapping leading to a lowering of the energy. Whereas, in the exo transition state there are no favourable interactions that result in a decreased HOMO-LUMO gap hence this transition state is of higher energy. This stereospecific observation is confirmed by Caramella et al. [1]
Figure 1: Illustration of Dimers:
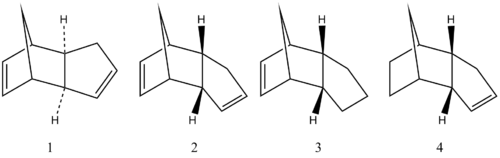
Hydrogenation of endo dimer 2 then leads to the formation of two more molecules labelled 3 and 4 in figure 1 respectively. Similarly to the comparison of dimers 1/2, the relative energies of dimers 3 and 4 were calculated and analysed via MM2 force field option in ChemBio3D. Overall from the calculations carried out, we would expect dimer 4 to be the most thermodynamically stable as it has a total energy of 31.1520 kcal/mol in comparison to 35.9266 kcal/mol for dimer 3. This observation is confirmed as the intermediate (named 9,10-Dihydrodicyclopentadiene) in the reaction proposed by Liu which suggests that the formation of 1,2-Dihydrodicyclopenatadiene (3) was neglected due to its low concentration; hence it can be concluded that 4 is the more stable intermediate.[2] From the data, it is clear that the major difference between 3 and 4 lie in the bend and stretch-bend energies. Dimer 3 has a noticeably more positive bend energy than 4 indicating a unfavourable interaction.
| Property | Dimer 1 | Dimer 2 | Dimer 3 | Dimer 4 |
|---|---|---|---|---|
| Stretch (kcal/mol) | 1.2851 | 1.2508 | 1.2349 | 1.0965 |
| Bend (kcal/mol) | 20.5805 | 20.8477 | 18.9384 | 14.5244 |
| Stretch-bend (kcal/mol) | -0.8381 | -0.8358 | -0.7609 | -0.5494 |
| Torsion (kcal/mol) | 7.6554 | 9.5109 | 12.1238 | 12.4974 |
| Non-1,4 VDW | -1.4173 | -1.5439 | -1.5016 | -1.0701 |
| 1,4 VDW (kcal/mol) | 4.2333 | 4.3201 | 5.7829 | 4.5126 |
| Dipole/Dipole (kcal/mol) | 0.3775 | 0.4476 | 0.1631 | 0.1406 |
| Total energy (kcal/mol) | 31.8764 | 33.9976 | 35.9266 | 31.1520 |
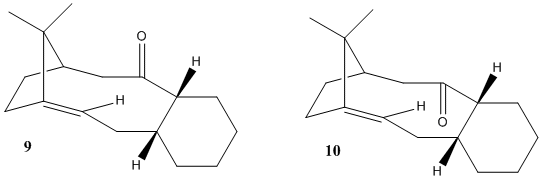
During the synthesis of Taxol, one of the steps includes a key intermediate whereby the carbonyl group can point either up or down, illustrated in figure 2. An investigation was carried out to explore which of the two atropisomers is more stable by calculating the energy via the MM2 force field in ChemBio3D and comparing this to optimising the geometry using the MMFF94(s) force field in the Avogadro program.[3] [4] Due to the nature of the molecule, the cyclohexane ring can form four different conformational isomers; two twist boats and two chair conformers. For atropisomer 9 twist boast A and B kcal/mol had energies of 53.0101 and 53.3046 kcal/mol respectively. In comparison, chair conformer A and B had respective energies of 47.8395 and 58.6458 kcal/mol. The chair conformation is the lowest energy conformation as we would expect and hence, chair conformer A was chosen to represent 9. For atropisomer 10 twist boast A and B kcal/mol had energies of 46.4336 and 48.1578 kcal/mol respectively. In comparison, chair conformer A and B had respective energies of 42.6829 and 52.5418 kcal/mol. The chair conformation is the lowest energy conformation as we would expect and hence, chair conformer A was chosen to represent 10.
The results of this investigation are shown in table 2. From the results, it can be seen that atropisomer 9 has a total energy of 47.8394 kcal/mol , in contrast, atropisomer 10 has a lower energy of 42.6829 kcal/mol. The difference between the two atropisomers lies in the bending energy, as the rest of the parameters have fairly similar values. This is due to the O-C-C bond angle being 115.7 o in 9 causing more steric hindrance whereas, 10 has a O-C-C bond angle of 120.0o therefore lower in energy. Hence, I would assume that 10 is the more thermodynamically stable of the two and would expect this isomer to be formed.[5] However, literature values could not be found for these molecules and so a direct comparison cannot be made with any accurate results.
| Property | MM2 (9) | MMFF94s (9) | MM2 (10) | MMFF94s (10) |
|---|---|---|---|---|
| Stretch (kcal/mol) | 2.7847 | 13.44709 | 2.6203 | 10.89319 |
| Bend (kcal/mol) | 16.5410 | 0.62344 | 11.3400 | 0.46553 |
| Stretch-bend (kcal/mol) | 0.4304 | 0.11016 | 0.3434 | -0.18525 |
| Torsion (kcal/mol) | 18.2512 | -1.51969 | 19.6672 | -2.00674 |
| Non-1,4 VDW (kcal/mol) | -1.5522 | N/A | -2.1588 | N/A |
| 1,4 VDW (kcal/mol) | 13.1092 | 36.17585 | 12.8730 | 36.41188 |
| Dipole/Dipole (kcal/mol) | -1.7248 | N/A | -2.0022 | N/A |
| Total energy (kcal/mol) | 47.8395 | 71.6051 | 42.6829 | 66.1783 |
From the data in table 2, it is clear that the total energy values produced vary significantly depending on the program and force field used. For both atropisomers, vary by approximately 24 kcal/mol this is roughly 50% of the energy calculated using the MM2 force field. Hence, it is difficult to to say the results are either accurate or reliable due to the range and also lack of literature values. However many attempts were undertaken to ensure the structure was in the optimum geometry, for example ensuring that the cyclohexane ring was in the chair conformation as this has the lowest energy.
Molecules 9 and 10 have usual reactivity for alkenes as they are part of the class of hyperstable alkenes. This is due to the alkene double bond being positioned at the bridgehead which reduces the reactivity of the molecule in comparison to the parent hydrocarbon equivalent. The two alkanes have been modeled here: parent hydrocarbon 9 and parent hydrocarbon 10. The parent hydrocarbons are destabilized due to the unfavourable transannular interactions between hydrogen atoms, making it more reactive and therefore higher in energy. Whereas the cage-like structure adjacent to the bridgehead alkene results in favourable VDW interactions, and hence stabilising 9 and 10. [6]
Spectroscopic Simulation using Quantum Mechanics
Molecules 17 and 18 are derivatives of molecules 9 and 10 described above. Using the data from the previous section where atropisomer 10 was found to be the most stable, the decision was made to perform the spectroscopic calculations on molecule 18 as shown in figure 3. The cyclohexane ring of 18 can orientate itself into several conformers, the two of which have been investigated are the boat and chair conformers which have relative energies of 61.7039 and 64.3642 kcal/mol respectively. However, it is expected that the lowest energy conformer should be a chair therefore it is clear that the absolute lowest energy conformer was not found.
The geometries of both of the conformers of 18 were optimised using Avogardo and the 1H and 13C NMR spectra were produced quantum mechanically using Gaussian using B3LYP/6-31G(d,p) simulation and in a solvent of benzene so that the results are comparable to the literature. The literature 1H NMR was ran at 300MHz and the 13C NMR was ran at 75MHz, both in C6D6. [7]
[8] The results of this investigation are shown in tables 3 and 4.
[9][10]
From the results of the 1H NMR shown in table 3 it can be seen that neither of the boat or chair conformers exactly match the literature data, however it seems the degeneracies of the boat signals are more comparable to that of the literature. The chemical shifts for the boat conformer are fairly close to that found by Paquette et al[8], differing by no more than ±0.25ppm for any of the signals. Whereas, the chair conformer chemical shifts cover a larger range from 5.97-0.60ppm in contrast to the literature which only covers a range from 5.21-1.03ppm. In addition, the degeneracies of those signals are much less closely matched to the literature differing by approximately ±0.76ppm at the most. By comparing the 1H NMR for the boat and chair conformers it can be concluded that the conformer of a molecule does affect the spectroscopic data significantly as protons are different chemical environment hence this affects the position of the chemical shift. For example, the least shielded chemical shift in the NMR differs by 0.61ppm between the two conformers which is a large difference. Overall it would seem that the close agreement between the QM calculated boat 1H NMR and the experimental data would suggest that the literature was based on a boat conformer of 18.

| Literature (ppm) | Boat Conformer (ppm) | Chair Conformer (ppm) |
|---|---|---|
| 5.21 (m,1H) | 5.36 (1H) | 5.97 (1H) |
| 3.00-2.70 (m, 6H) | 3.33 (1H) | 3.15-3.01 (2H) |
| 2.70-2.35 (m, 4H) | 3.09 (1H) | 2.95 (2H) |
| 2.20-1.70 (m, 6H) | 3.03 (1H) | 2.89 (1H) |
| 1.58 (t, 1H) | 2.93 (1H) | 2.82-2.78 (2H) |
| 1.50-1.20 (m, 3H) | 2.79 (1H) | 2.67(1H) |
| 1.10 (s, 3H) | 2.60 (1H) | 2.56-2.52 (2H) |
| 1.07 (s, 3H) | 2.51 (1H) | 2.43 (1H) |
| 1.03 (s, 3H) | 2.45 (1H) | 2.33-2.30 (2H) |
| 2.22-2.18 (2H) | 2.00-1.96 (2H) | |
| 2.07 (2H) | 1.85-1.81 (2H) | |
| 1.98-1.93 (3H) | 1.64 (1H) | |
| 1.84-1.81 (2H) | 1.57-1.50 (3H) | |
| 1.73 (1H) | 1.34 (1H) | |
| 1.64 (1H) | 1.27 (1H) | |
| 1.55-1.51 (2H) | 1.20 (2H) | |
| 1.39 (1H) | 0.97 (2H) | |
| 1.30 (1H) | 0.90 (1H) | |
| 1.16(1H) | 0.60 (1H) | |
| 1.08-0.95 (5H) |
In the 13C NMR the chemical shifts of the carbon atoms adjacent to sulphur atoms have been corrected due to spin-orbit coupling errors. Typically, C-Cl bonds need to be corrected by -3ppm and hence because sulphur is of a similar size to chlorine (next to each other in the periodic table) then the corresponding signals for C-S bonds were also corrected by -3ppmm.[11] The corrected chemical shifts for the boat conformer are: 85.68, 41.29 and 38.24ppm. Similarly, for the chair conformer the corrected chemical shifts are: 89.84, 41.01 and 38.47ppm. By comparison of the calculated 13C NMR for the boat conformer and the literature[8] it can be seen that most of the signals are closely matched, differing by less than 4ppm in general. Similarly, for the chair conformer there is some agreement with the chemical shifts of the literature, however this is less predominant than the boat. One exception to this observation, is the signal in the literature appearing at 74.61ppm as this is significantly different from either of the calculated NMR data. The difference is greater than 10ppm and hence this cannot be concluded as a good agreement, which suggests the possibility that Paquette[8] may have reported this particular chemical shift incorrectly.
| Literature (ppm) | Boat Conformer (ppm) | Chair Conformer (ppm) |
|---|---|---|
| 211.49 | 211.02 | 211.92 |
| 148.72 | 148.57 | 147.87 |
| 120.90 | 118.62 | 120.12 |
| 74.61 | 85.68 | 89.84 |
| 60.53 | 67.54 | 65.94 |
| 51.30 | 56.08 | 54.94 |
| 50.94 | 55.40 | 54.76 |
| 45.53 | 49.92 | 49.53 |
| 43.28 | 46.59 | 48.03 |
| 40.82 | 41.29 | 45.65 |
| 38.73 | 38.24 | 41.01 |
| 36.78 | 37.45 | 38.47 |
| 35.47 | 36.16 | 38.51 |
| 30.84 | 31.99 | 33.69 |
| 30.00 | 29.09 | 32.47 |
| 25.56 | 28.62 | 28.36 |
| 25.35 | 25.85 | 26.50 |
| 22.21 | 25.10 | 24.44 |
| 21.39 | 23.28 | 24.01 |
| 19.83 | 21.70 | 22.58 |
References
- ↑ P. Caramella, P. Quadrelli and L. Tomo, "An Unexpected Bispericyclic Transition Structure Leading to 4+2 and 2+4 Cycloadducts in the Endo Dimerization of Cyclopentadiene", J. Am. Chem. Soc., 2002, 124(7), 1130–1131.DOI:10.1021/ja016622h
- ↑ G. Liu , Z. Mi , L. Wang , and X. Zhang, "Kinetics of Dicyclopentadiene Hydrogenation over Pd/Al2O3 by catalyst", Ind. Eng. Chem. Res., 2005, 44(11), 3846-3851.DOI:10.1021/ie0487437
- ↑ See J. G. Vinter and H. M. R. Hoffman, J. Am. Chem. Soc., 1974, 96, 5466–5478 (DOI:10.1021/ja00824a025
- ↑ Force Fields in Avogadro
- ↑ S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319; DOI:10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0
- ↑ W. F. Maier, P. Von Rague Schleyer, J. Am. Chem. Soc., 1981, 103, 1891. DOI:10.1021/ja00398a003
- ↑ L. Paquette, K. D. Combrink, S. Elmore and R. Rogers, J. Am. Chem. Soc., 1991, 113(4), 1335-1344. DOI:10.1021/ja00004a040
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 L. Paquette, N. Pegg, D. Toops, G. Maynard and R. Rogers, J. Am. Chem. Soc., 1990, 112(1), 277-283. DOI:10.1021/ja00157a043
- ↑ L. Theodoulidis, "Taxol 18 chair conformation NMR", DSpace, 2013,DOI:10042/25830
- ↑ L. Theodoulidis, "Taxol 18 boat conformation NMR", DSpace, 2013,DOI:10042/25831
- ↑ C. Braddock and H. S. Rzepa, J. Nat. Prod., 2008, 71, 728-730. DOI:10.1021/np0705918
Experiment 1C: Part 2
Analysis of the properties of the two synthesised alkene epoxides
Crystal structures of Shi and Jacobsen pre-catalysts
The Shi catalyst is an asymmetric fructose-based catalyst which has been proven to effectively catalyse the epoxidation for trans- or trisubstituted alkenes. [1] One of the stable precursors to the Shi catalyst is species 21 which is illustrated in the Jmol below. The Cambridge crystal database was searched using the Conquest program for 21. This was then transferred to Mercury to analyse and to compare the C-O bond lengths at the two anomeric centres. It is clear from the Jmol that there is a change in bond length at the anomeric centre. Whilst the anomeric centre based in the cyclohexane ring has two equal C-O bond length of 0.14nm, the other anomeric centre in the cyclopentane ring has bond lengths of 0.144 and 0.141nm respectively. This is due to the orientation of the anomeric centre, the oxygen in the cyclohexane ring is equitorial and therefore cannot undergo hyperconjugation to delocalise its lone pair into the C-O bond, due to the lack of an anti-periplanar arrangement, hence why the C-O bonds at this anomeric centre are equal. Whereas, the oxygens in the cyclopentane ring are in an anti-periplanar arrangmeent and so hyperconjugation occurs to delocalise the oxygen lone pairs into the σ* anti-bonding orbital of the C-O bond. As a result of the increase in electron density in the anti-bonding orbital, the bond weakens and the bond length increases. The crystal structure of the pre-catalyst 21 can also be used to explain the enantioselectivity of the Shi catalyst. When persulphuric acid is added to the stable pre-catalyst 21 the active catalyst is formed and the carbonyl is replaced with a dioxirane ring.[2] This active catalyst then has two five-membered rings located above and below the six-membered ring. As a result, trans-alkenes are favoured in comparison to cis-alkenes due to lack of steric hinderance when approaching the dioxirane ring.
Shiprecusor21 |
The other catalyst that will be used in experiment 1S is the asymmetric Mn-based Jacobsen catalyst. In contrast to Shi, the Jacobsen enantioselectively catalyses the epoxidation of cis-alkenes to form cis-epoxides. [3] In this experiment, a stable precursor of the Jacobsen catalyst was investigated on the Cambridge crystal database. A 3D structure of this precursor which we will refer to as 23 is shown in a Jmol below. The distance between the two adjacent t-butyl groups is shown below as 0.242nm, measured between the two approaching hydrogen atoms. This is in the region for van der Waals attraction and so stabilises the molecule. These t-butyl groups, in addition to the other t-butyl groups bonded to the phenyl ring, block the side-on approach of the alkene to the metal-oxo bond. This results in the alkene approaching from above the diimine bridge, and hence giving a cis-selective epoxidation due to sterics. This is referred to as approach d in Jacobsen et al. [3]
Jacobsen23 |
NMR properties of epoxide products
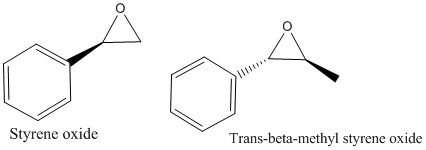
Figure 4: Illustration of Epoxides:
The two alkenes styrene and trans-β-methyl styrene oxide were chosen to base a theoretical epoxidation reaction on. The two predicted products of these reactions are shown in figure 4. The structures were drawn on ChemBio3D and then optimised using Avogardo software before the NMR being measured on Gaussian using B3LYP/6-31G(d,p) simulation. Both of the calculated 1H and 13C NMR for (R)-styrene oxide were compared to the same literature where 1H was carried out at 400 MHz and CDCl3 and 13C NMR at 100 MHz also in CDCl3. [4] The results obtained for (R)-styrene oxide are shown in table 5.[5] In general, the calculated 1H chemical shifts agree with the experimental values, varying by around ±0.2ppm. The first two chemical shifts for the calculated NMR at 7.51-7.45ppm and 7.30ppm correspond to the 5H multiplet between 7.33-7.24ppm relating to the aromatic protons in the benzene ring. Although the H-H coupling constants were not measured using QM, as this calculation would take over 24 hours, we would expect that the J values would be in agreement with the literature. The largest 2JH-H coupling constant of 5.6Hz corresponds to the two hydrogens on the same carbon atom, adjacent to the epoxide. One of these hydrogens then couples with the trans-hydrogen on the adjacent carbon resulting in 2JH-H=4.1Hz and the final coupling constant 2JH-H=2.8 Hz corresponds to the two cis-hydrogen atoms on either side of the epoxide coupling.

By comparison of the calculated 13C data with the literature we can see that there is a close correlation between the two, with differences varying between 2-7ppm. The signal in the literature at 128.2ppm which is a doubly degenerate signal must correspond to the two signals at 124.14 and 124.41ppm peaks calculated. The peak at 118.27ppm is much more shielded that the equivalent peak in literature which is at 125.5ppm. This may be due to external experimental factors that the quantum mechanical calculations does not take into account.
| Literature 1H NMR (ppm) | Calculated 1H NMR (ppm) | Literature 13C NMR (ppm) | Calculated 13C NMR (ppm) |
|---|---|---|---|
| 7.33-7.24 (5H, m) | 7.51-7.45 (4H) | 137.5 | 135.13 |
| 3.80 (1H, dd, J=2.8,4.1 Hz) | 7.30 (1H) | 128.2 (2C) | 124.14 |
| 3.08 (1H, dd, J=4.1,5.6 Hz) | 3.66 (1H) | 128.0 | 123.41 |
| 2.75 (1H, dd, J=2.8,5.6 Hz) | 3.12 (1H) | 125.5 (2C) | 122.97 (2C) |
| 2.54 (1H) | 51.1 | 118.27 | |
| 51.0 | 54.06 | ||
| 53.48 |


The calculated data for 1H and 13C NMR of (S,S)-trans-β-methyl styrene oxide were both compared to literature 1H(400MHz,CDCl3) and 13C (100MHz, CDCl3). [6] The results are shown in table 6.[7] Similarly to (R)-styrene oxide, the calculated 1H NMR data has a much larger range than the literature. In this case, the literature chemical shifts range from 7.25ppm down to 1.43ppm whereas the calculated NMR ranges from 7.50-0.72ppm. There is a good agreement for the chemical shift of the aromatic protons, in contrast however, the most shielded proton at 0.72ppm is significantly lower than the literature by approximately 0.7ppm.
On the other hand, the 13C NMR that was calculated using QM has a much better agreement with literature than the 1H data. By comparing the data, the largest disagreement is between 118.50ppm peak in the calculated NMR and the literature which is 125.4ppm. The literature data provided in the table, isnt a perfect match to the simulated NMR as there appears to be less C atoms than anticipated, this is most likely due to doubly degenerate peaks which have not been reported in the paper. However, in general there is a good match between data sets to within approximately 5ppm. Although this confirms the structure, it does not give any information on the absolute configuration of the product i.e. which enantiomer is in excess, further measurements are required to find this. It is assumed that both enantiomers of styrene oxide and trans-β-methyl styrene oxide will have identical NMR spectra and hence NMR was only computed for one of the enantiomers in each case.
| Literature 1H NMR (ppm) | Calculated 1H NMR (ppm) | Literature 13C NMR (ppm) | Calculated 13C NMR (ppm) |
|---|---|---|---|
| 7.25 (5H, m) | 7.50-7.48 (3H) | 137.7 | 134.98 |
| 3.55 (1H, d, J=2.5 Hz) | 7.42 (1H) | 128.3 | 124.08 |
| 3.12 (1H, dq, J=2.5, 6.8 Hz) | 7.31 (1H) | 127.9 | 123.33 |
| 1.43 (3H, d, J=6.8 Hz) | 3.41 (1H) | 125.4 | 122.73 (2C) |
| 2.79 (1H) | 59.4 | 118.50 | |
| 1.68 (1H) | 58.9 | 62.31 | |
| 1.59 (1H) | 17.8 | 60.57 | |
| 0.72 (1H) | 18.86 |
Assigning the absolute configuration of the products
The objective of this task is to assign the absolute configuration of the product obtained using either the Shi or Jacobsen catalyst as the NMR information alone will not provide this. This can be done by measuring the chiroptical properties of a specific enantiomer (drawn in ChemBio3D) and comparing this information with the experimentally measured values to predict the enantioselectivity of the catalyst.
The styrene oxide enantiomer drawn in ChemBio3D was assigned the configuration (R)-styrene oxide. The optical rotatory power for this particular enantiomer was then measured quantum mechanically at 589nm and 365nm which resulted in optical rotations of -30.39 deg and -94.88 deg respectively. [8] This was then compared to literature data found using Reaxys. A literature comparison for the optical rotatory power (ORP) measured at 365nm could not be found and so this was neglected. However, Lin et al. reported a ORP of +30.2 deg for the (S)-styrene oxide.[9] This value is extremely close to the simulated ORP, equal but opposite hence this confirms that the enantiomer drawn was indeed the R enantiomer. Lin et al' also reported that the was a >99% enantiomeric excess (ee) hence it would be predicted that during the experiment, the (S)-styrene oxide would be in substantial excess.
A similar process was then carried out for trans-β-methyl styrene oxide which was assigned the stereochemistry (1S,2S) using ChemBio3D. The optical rotatory power for this particular enantiomer was then measured quantum mechanically at 589nm and 365nm which resulted in optical rotations of -47.46 deg and -140.24 deg respectively. [10] Again, no literature value for the ORP at 365nm was found to compare to, so this was neglected. Besse et al. reported that the (S,S)-trans-β-methyl styrene oxide had an ORP of -42 deg.[11] The slight difference in values by 5 deg may be due to Besse et al using yeast as a catalyst, instead of the Shi catalyst that we will be using in experiment 1S. Alternatively, the difference may be due to the enantiomer drawn in ChemBio3D not being at its most optimised, or lowest in energy configuration.
Using the (calculated) properties of transition state for the reaction (β-methyl styrene only)
The free energies of the transition states for the reaction of β-methyl styrene only (both cis and trans) were computed and used to predict the selectivity of the catalysts. This was done by identifying the total energy for each system as corrected for entropy and zero-point thermal energies and comparing to find the lowest energy transition state. The change in free energy (ΔG) was then calculated between the two diastereomeric transition states. Then using the equation below, the ratio of concentrations of the two species K can be measured.
The equation above is ΔG=-RTlnK rearranged in terms of K.
The transition states for the Shi epoxidation of trans-β-methyl styrene were compared and it was founded that the lowest energy transition state corresponded to the phenyl group of the alkene being orientated endo with respect to the fructose. The energy values were converted from hartree to kJ/mol and the results of these calculations are shown in table 7. For trans-β-methyl styrene the ratio of concentration of the two enantiomers (R,R/S,S) based on energy indicates that the the (R,R) enantiomer is significantly favoured due to the large K value of 3486.652116. This leads to an enantiomeric excess of 99.97%. Shi et al confirm this observation with their paper reporting they carried out the Shi epoxidation with 88% ee of the (R,R)-trans-β-methyl styrene oxide.[12] Although this is in close agreement with the results simulated by Gaussian, a direct comparison cannot be made as Shi et al recorded the results in chloroform as a solvent whereas the simulated results were computed in water.
| ΔG for trans-β-methylstyrene epoxidation (kJ/mol) | K for trans-β-methylstyrene | ΔG for cis-β-methylstyrene epoxidation (kJ/mol) | K for cis-β-methylstyrene |
|---|---|---|---|
| -20.218977 | 3486.652116 | -22.3141262 | 8118.640311 |
In the case of the Jacobsen epoxidation of cis-β-methyl styrene, the endo transition state is lower in energy than the exo transition state and so these free energies were used to calculate ΔG shown in table 7. The K ratio (S,R/R,S) for this epoxidation is also extremely high at 8118.640311, implying the S,R enantiomer is dramatically favoured. The S,R enantiomer is favoured with 99.98% ee. Jacobsen et al confirm this observation reporting a 92% ee of the (S,R)-cis-β-methyl styrene oxide. [13]
Investigating the non-covalent interactions in the reaction transition state
Orbital |
Non-covalent interactions (NCI) include hydrogen bonds, electrostatic attractions and dispersion-like close approaches of pairs of atoms. NCI analysis was carried out on one of the (R,R) endo transition states (the one with the highest energy) of trans-β-methyl styrene oxide formation, using Gaussian software and the result is shown in the Jmol above. The colour green indicates a mildly attractive interaction. For example this attraction is predominant between the Shi catalyst and the alkene transition state as the two molecules approach each other for the epoxidation reaction to occur. In some areas, for example between the oxygen atoms in the 5-membered ring, a red isosurface can be seen. This implies a strongly repulsive interaction most likely due to the lone pairs on each oxygen atom repelling one another as they are in such close proximity. In addition, there is a ring of red and blue situated between the dioxirane ring of the Shi catalyst and the alkene bond of trans-β-methyl styrene. This is a result of the oxygen lone pair causing repulsive interactions, but also the strongly attractive interactions (indicated by the blue isosurface) are due to the favourable formation of the product, which is much lower in energy. However, the attractive NCI greatly outweigh the repulsive interactions hence why the transition state is stable.
Investigating the electronic topology (QTAIM)in the active-site of the reaction transition state
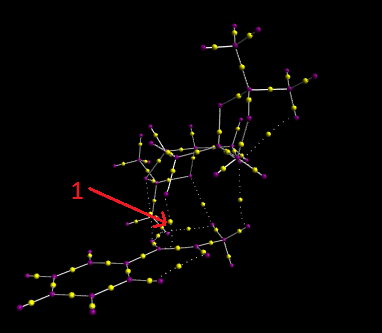
The Quantum Theory of Atoms in Molecules (QTAIM) algorithm was then carried out on high energy exo (R,R)-trans-β-methyl styrene transition state. QTAIM defines the chemical bonding and structure of a chemical system based on the topology of the electron density. The results of this investigation are shown in the screenshot above. The yellow dots in a sold bond indicate a topological point known as a bond critical point (BCP), which is a region where the first derivative of the electron density with respect to each of the three coordinates of that point is zero. Whereas, the yellow dot located on a dotted line represent a weak non-covalent BCP which is associated with a weak interaction between an oxygen and a hydrogen atom.
In general we can see that the covalent BCPs lie closer to the more electropositive atom e.g. in a C-H bond the BCP lies close to the hydrogen. One of the more interesting BCPs that can be seen is the weak non-covalent BCP, illustrated by arrow 1, between the C=C bond in the alkene and the dioxirane ring in the Shi catalyst. This shows the beginning of the covalent bond which will allow the formation of the epoxide.
Suggesting new candidates
By searching Reaxys, another possible candidate for this experiment named methyl-(3,4-anhydro-β-L-ribopyranoside) was found to have a ORP of 542.8deg at 589nm. [14] The structure of this candidate is shown here. Although the molecule has a lot of functionality, with the epoxide, ether and alcohol the structure is fairly simple hence it is expected that the alkene precursor is commercially available. In addition, the compound has very few references and is unlikely to have had its absolute configuration confirmed by QM computation before. An interesting investigation would be to compute the ORP and compare this to the literature assignment.
References
- ↑ O. A. Wong , B. Wang , M-X Zhao and Y. Shi J. Org. Chem., 2009, 74, 335–6338; DOI:10.1021/jo900739q
- ↑ A. Burke , P. Dillon , Kyle Martin and T. W. Hanks,"Catalytic Asymmetric Epoxidation Using a Fructose-Derived Catalyst", J. Chem. Educ., 2000, 77, 271; DOI:10.1021/ed077p271
- ↑ 3.0 3.1 E. N. Jacobsen , W. Zhang , A. R. Muci ,J. R. Ecker , L. Deng, J. Am. Chem. Soc., 1991, 113, 7063–7064; DOI:10.1021/ja00018a068
- ↑ 4.0 4.1 C. Wiles , M. J. Hammond and P. Watts, "The development and evaluation of a continuous flow process for the lipase-mediated oxidation of alkenes", Beilstein J. Org. Chem, 2009, 5(27), 1-12.DOI:10.3762/bjoc.5.27
- ↑ L. Theodoulidis, "(R)-Styrene oxide NMR", DSpace, 2013,DOI:10042/25833
- ↑ 6.0 6.1 H. Hachiya, Y. Kon, K. Takumi, N. Sasagawa, Y. Ezaki, and K.Sato, "Unique Salt Effect on Highly Selective Synthesis of Acid-Labile Terpene and Styrene Oxides", Synthesis, 2012, 44 (11), 1672-1678. DOI:10.1055/s-0031-1290948
- ↑ L. Theodoulidis, "(1S,2S) Trans-β-methyl styrene oxide NMR", DSpace, 2013,DOI:10042/25834
- ↑ L. Theodoulidis, "(R)-Styrene oxide ORP", DSpace, 2013,DOI:10042/25835
- ↑ H. Lin, J. Qiao, Y. Liu, Z. Wu, "Chemoenzymatic synthesis of chiral epoxides. Preparation of 4-phenyl-2,3-epoxybutane and 1-phenyl-1,2-epoxypropane", Tetrahedron, 1994, 5(7), 236-241. DOI:10.1016/j.molcatb.2010.08.012
- ↑ L. Theodoulidis, "(1S,2S)-trans-β-methyl styrene oxide ORP", DSpace, 2013,DOI:10042/25836
- ↑ P. Besse, M. Renard, H. Veschambre, "Asymmetric epoxidation of both conjugated and unconjugated alkenes", Journal of Molecular Catalysis B, 2010, 67 (3-4), 1249-1268. DOI:10.1016/0957-4166(94)80167-3
- ↑ Z. Wang, Y. Tu, M. Frohn, J. Zhang and Y. Shi,"An Efficient Catalytic Asymmetric Epoxidation Method", J. Chem. Educ., 1997, 119(46), 11224-11235; DOI:10.1021/ja972272g
- ↑ E. Jacobsen, W. Zhang, A. Muci, J. Ecker and L. Deng,"Highly enantioselective epoxidation catalysts derived from 1,2-diaminocyclohexane", J. Chem. Educ., 1991, 113(18), 7063-7064; DOI:10.1021/ja00018a068
- ↑ N. Mohal and A. Vasella, "Synthesis of Fusion-Isomeric Imidazopyridines and Their Evaluation as Inhibitors of syn- and anti-Protonating Glycosidases", Helvetica Chimica Acta, 2005, 88(1), 100-119. DOI:10.1002/hlca.200490287