Rep:Mod:LOTR1
Modelling using Molecular Mechanics
The Hydrogenation of Cyclopentadiene Dimer
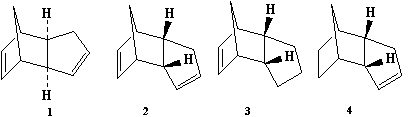
The dimerisation of cyclopentadiene affords the exo dimer 1 and endo dimer 2, where the endo dimer is the major product. This can be rationalised by molecular dynamics using MM2 force field to compare relative stabilities of the two dimers. The total energy calculated is 34.0 kcal/mol (142.4 kJ/mol) for the exo dimer and 31.9 kcal/mol (133.5 kJ/mol) for the endo dimer. Therefore the endo dimer proceeds via thermodynamic control as it is less strained giving product stability and forms the major product, whereas the exo dimer is kinetically controlled which gives transition state stability.
Energy calculations of the two products of hydrogenation 3 and 4 are as follows:
 |
 |
| Energy terms (kJ/mol) | 3 | 4 |
|---|---|---|
| Total | 150.5 | 130.5 |
| Stretch | 5.1 | 4.6 |
| Bend | 79.1 | 60.8 |
| Torsion | 50.8 | 52.4 |
| Van der Waals | 10.9 | 12.5 |
From the total energy calculated the dihydro derivative 4 is seen to be the more thermodynamically stable product and hydrogenation of the double bond is relatively easier than the other double bond hydrogenated in 3. Greatest contribution of relative stabilities are given by the bending and torsion energy term, where 3 has greater strain by bending compared to 4, but torsional strain is greater for 4. However small contributions of stretching and Van der Waals energy is lower for 4 compared to 3 giving greater product stability and so 4 is the thermodynamically controlled product.
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
The reaction of methyl magnesium iodide with the optically active derivative of prolinol to give absolute stereoselectivity for the alkylation of the pyridine ring in the 4-position, can be explained by modelling the pyridinium reactant. Minimising the models using the MMFF94 force field reveals two conformational minimas for changing the conformation of the 7-ring, which has differing geometry for the amide carbonyl group and its orientation with respect to the pyridine ring system.

|
 |

The first pyridinium conformer is seen to have the amide carbonyl group above the plane of the pyridine ring with a calculated dihedral angle of 37° and is antiperplanar to the hydrogen atom at the chiral carbon, whilst the other pyridinium conformer with the chiral carbon moved up and its adjacent carbon in the 7-ring moved down, has the carbonyl group mostly coplanar with the pyridine ring, with a dihedral angle of 3°. Relative energies of the conformers were calculated to be 59.1 kcal/mol (247.6 kJ/mol) for the first conformer and 57.5 kcal/mol (240.7kJ/mol) for the second conformer indicating the coplanar arrangement of the carbonyl group and pyridine ring is the lowest energy conformation.
The mechanism is the MeMgI coordinates to the amide oxygen first and there is conjugate delivery of the methyl group from the Magnesium to the C4 in the pyridine ring to give a magnesium enolate. Since there are no conformations where the amide carbonyl group is below the plane of pyridine ring, which would give the methyl group below the pyridine ring, instead there is complete stereoselectivity for the addition of grignard reagents to the pyridinium reactant so the methyl group only forms above the plane of the pyridine ring.[1]
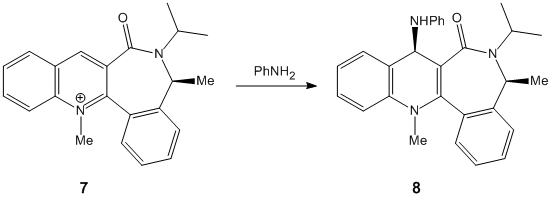
Pyridinium reactant 7 reacts with aniline also with complete stereoselectivity to give 8 with the NHPhenyl group above the plane of the pyridine ring.
 |
|
 |
Minimisation of 7 gives two conformational minima, one with the amide carbonyl group above the plane of the pyridine ring with a calculated dihedral angle of 37° and one with the amide carbonyl group below the plane of the pyridine ring with a calculated dihedral angle of -38°. Aniline attacks the pyridine ring at 4-position with diastereofacial selectivity due to the steric control exerted by the carbonyl forcing the aniline to attack on the opposite face with respect to the amide carbonyl group. The relative energies of the conformers are 113.2 kcal/mol (474.1 kJ/mol) for the carbonyl group above the plane of the pyridine ring and 98.4 kcal/mol (411.9 kJ/mol) for the carbonyl group below the plane of the pyridine ring. Therefore the amide carbonyl group below the plane of the pyridine ring conformer is the preferred conformation and so aniline attacks above the plane of the pyridine ring giving the NHPhenyl group also above the plane of the pyridine ring. [2]
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
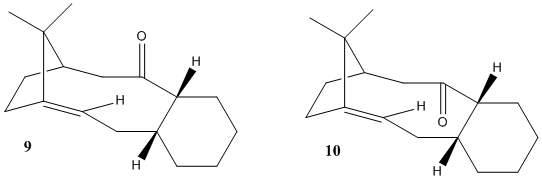
The intermediate in the synthesis of taxol 9 with the carbonyl group pointing upwards was minimised using the MM2 force field to give two conformers with different conformations of the C-6 ring. This is the chair and twist boat conformations where the chair conformation of 9 is more stable as can be seen by their relative energies.
Rotation of the carbonyl group into 10 also gives two conformers of the chair and twist boat conformation of the C-6 ring, with the chair conformation lower in energy than the twist boat. The relative energies of the 10 conformers are lower than the relative energies of the 9 conformer, so 10 is the more stable conformer with the 10 chair conformation being the most stable. [3]
| 9 twist boat | 9 chair | 10 twist boat | 10 chair | |
|---|---|---|---|---|
| Energy terms (kJ/mol) | 226.5 | 204.7 | 212.3 | 185.5 |
Upon standing, 9 isomerises to 10 chair rather than rapidly inter-converting due to the restricted motion of the C-C single bond adjacent to the carbonyl, where the conformation of the C-6 ring locks the carbonyl in either upwards or downwards positions.
The alkene in the taxol intermdiate reacts slowly because strain energy of the alkene is lower than that of the corresponding alkene due to the increase in vicinal and trans-annualar hydrogen interactions in the alkane. This hyperstability is the cause of slow hydrogenation for the subsequent alkene. [4]
 |
 |
 |
 |
Modelling Using Semi-empirical Molecular Orbital Theory
Regioselective Addition of Dichlorocarbene
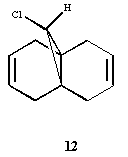
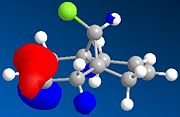
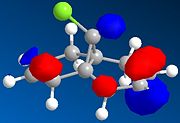
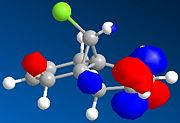
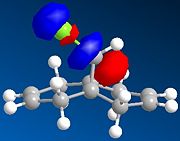
The regioselective reaction of compound 12 with electrophilic reagents, such as dichlorocarbene, can be explained by orbital control of reactivity. This is shown in modelling 12 to optimise the geometry, calculate the energy of the orbitals and displaying them graphically by using MM2 force field first and then MOPAC/PM6 MO methods.
Dichlorocarbene reacts regiospecifically on the C=C double bond endo to chlorine substituent because of attributing stabilisation of antiperiplanar interactions between the C-Cl σ* oribtal of the LUMO+1 and the exo π-orbital of the HOMO-1.[5] This stabilisation effectively makes the endo C=C double bond more nucleophilic with greater electron density in HOMO at the endo double bond and so is more reactive to electrophilic attack, hence regioselective.
 |
 |
 |
 |
 |
| Diene 12 | Monoalkene | ||
| Cl-C stretch | 689.5 | 698.4 | |
| exo C=C stretch | 1737.12 | - | |
| endo C=C stretch | 1757.3 | 1758.1 |
The vibrational frequencies of 12 were calculated using the density functional approach, where the frequency characteristic of Cl-C stretch is observed at 689.5cm-1, and 1737.1cm-1 and 1757.3cm-1 correspond to the exo C=C and endo C=C stretches respectively. The exo C=C stretching frequency is lower than the endo C=C indicating the exo C=C is weaker and is rationalised by the donation of electron density from the exo π-orbital to the C-Cl σ* oribtal, hence weakening the exo C=C bond. For the monoalkene the Cl-C stretch was observed at 698.4cm-1 and the endo C=C on Cl side at 1758.1cm-1. The Cl-C stretching frequency is lower for diene than the monoalkene, showing the Cl-C bond is weaker in the diene compared to the monoalkene. This is due the Cl-C σ* oribtal in the diene accepting electron density from the exo π-orbital which weakens the Cl-C bond but the monoalkene does not have a exo C=C bond to donate electron density, so the Cl-C bond is not weakened.
Structure based Mini project using DFT-based Molecular orbital methods
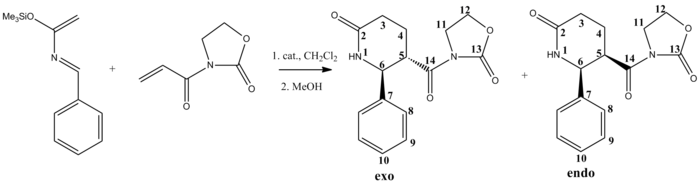
The reaction I have chosen to study is an asymmetric Diels-Alder reaction of a 2-azadiene and an iminodienophile in the presence of a chiral Lewis acid to yield two diastereomers, exo and endo.[6] The reaction scheme for my chosen reaction can be seen below.
|
 |
 |
Drawing the two isomers in ChemBio3D and minimising using MM2 force field gave relative energies of 29.5 kcal/mol for the exo isomer and 31.4 kcal/mol for the endo, which indicates the reaction is under thermodynamic control and exo diastereomer is the more stable conformation and agrees with literature to be the preferred conformer.
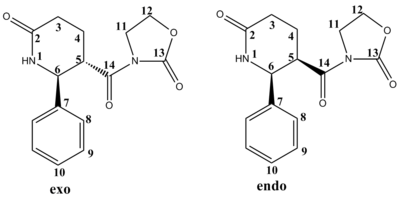
Calculated 13C NMR spectrum
| C atom | Exp δ/ppm | Calc δ/ppm |
|---|---|---|
| C14 | 171.48 | 163.61 |
| C2 | 170.76 | 161.62 |
| C13 | 152.96 | 146.73 |
| C7 | 138.79 | 136.90 |
| C8&C10 | 128.41 | 125.98 |
| C9 | 126.66 | 123.92 |
| C12 | 61.92 | 60.68 |
| C6 | 56.94 | 59.93 |
| C11 | 42.42 | 57.88 |
| C5 | 41.82 | 45.65 |
| C3 | 29.22 | 32.77 |
| C4 | 20.04 | 24.23 |
| C atom | Exp δ/ppm | Calc δ/ppm |
|---|---|---|
| C14 | 172.22 | 165.922 |
| C2 | 170.71 | 163.93 |
| C13 | 152.69 | 145.68 |
| C7 | 139.73 | 236.50 |
| C8 | 128.75 | 125.54 |
| C10 | 128.55 | 125.14 |
| C9 | 127.24 | 124.39 |
| C12 | 61.81 | 61.57 |
| C6 | 59.04 | 60.09 |
| C11 | 42.38 | 44.52 |
| C5 | 45.49 | 45.31 |
| C3 | 30.25 | 29.62 |
| C4 | 24.56 | 27.27 |
The predicted 13C NMR was calculated for the exo and endo isomers using the GIAO method. Comparing the predicted chemical shifts to literature, it can be seen there is a varying difference for different ppm values. The most chemically shifted carbonyl C-atoms (C14,C2&C13) have a difference of about 7ppm, which is not very precise, whilst the carbon atoms in the aromatic ring give a small chemical shift difference within 2ppm and could be considered to match literature, and the least chemically shifted carbon atoms in the lactam rings displays reasonable differences of 3/4 ppm. From the 13C NMR data it is difficult to distinguish between the two isomers as the chemical shifts are quite similar,even though the exo conformer is slightly greater chemically shifted compared to the endo isomer.

In terms of frontier orbitals, the interaction of the HOMO of the 2-azadiene with the LUMO of the iminodienophile in the exo approach where the diene attacks below the plane of the dienophile selectively gives the COR group down with respect to the phenyl group up in the six-membered lactam ring. This reduces the steric hinderence between the phenyl and bulky COR group as they point in different directions whereas both facing the direction as in the endo isomer.
References
- ↑ A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838.
- ↑ S. Leleu, C.; Papamicael, F. Marsais, G. Dupas, V.; Levacher, Vincent. Tetrahedron: Asymmetry, 2004, 15, 3919-3928.
- ↑ S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319.
- ↑ P. Camps, X. Pujol, S. Vasquez, M. A. Pericas, C Puigjaner and L. Sola, Tetrahedron, 2001, 8511-8520
- ↑ B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447.
- ↑ E. Jnoff, L. Ghosez, J. Am. Chem. Soc., 1999, 121, 2617-2618.
