Rep:Mod:LIUXYLANA1
Week 1:
Modelling using Molecular Mechanics
The Hydrogenation of Cyclopentadiene Dimer
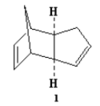
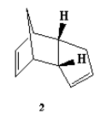
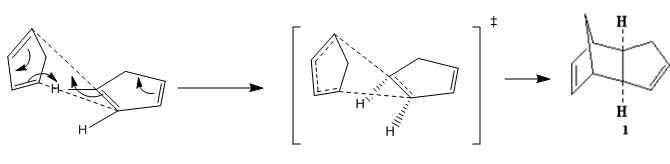
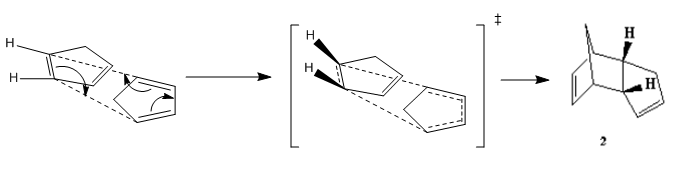
At room temperature, cyclopentadiene readily forms dimers which follows a concerted [4+2] cycloaddition mechanism where one of the cyclopentadiene acts as a dienophile and the other cyclopentadiene acts as a alkene, and produce two stereoisomers(1&2), where 1 is the exo-isomer and 2 is the endo-isomer( endo and exo are descriptions of orientations of substituents in transition states).
The reaction mechanism was shown as follow:
Molecular mechanic MM2 force field calculations are performed to determine the dominant effect(thermodynamic effect vs kinetic effect) in the formation of cyclopentadiene dimer.
Results present as follow:
| Energy Terms / kcal mol-1 | Exo-isomer Jmol | Endo-isomer Jmol | Difference in energy (Exo-Endo) |
| Stretch | 1.2852 | 1.2505 | 0.0347 |
| Bend | 20.5803 | 20.8482 | -0.2679 |
| Stretch-Bend | -0.8381 | -0.8356 | -0.0025 |
| Torsion | 7.6552 | 9.5107 | -1.8555 |
| Non-1,4 Vdw | -1.4173 | -1.5437 | 0.1264 |
| 1,4 Vdw | 4.2335 | 4.3198 | -0.0863 |
| Dipole/ Dipole | 0.3775 | 0.4476 | -0.0701 |
| Total Energy | 31.8765 | 33.9975 | -2.121 |
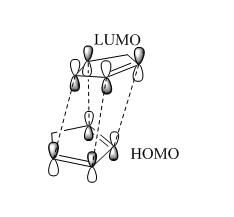
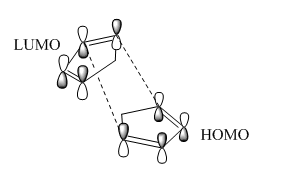
MM2 calculation shows that the total energy of endo-isomer is higher than the exo-isomer (by 2.121 kcal mol-1). This means that exo-isomer is more thermodynamically favoured. However, experimentally, more endo product is formed during the reaction at room temperature. This can be rationalised using Frontier Orbital Theory(Woodward and Hoffman[1]). At the transition state, 4 sets of HOMO-LUMO orbital bonding interactions were observed for the endo transition state where as only 2 sets of bonding interactions were found for the exo TS. Hence, the endo TS is lower in energy as compared to the exo TS which essentially means a lower activation barrier.
Hence, the reaction is dominated by kinetic effect(irreversible). However, given the endo isomer is the less stable form of product, provided with enough energy(180-240oC[2]), thermal isomerisation will take place, making exo isomer the leading product.
For both exo and endo dicyclopentadiene, 'Bend' contributes the most to their total energy, indicating a significant difference as compared to the optimum bond angle. The endo-dimer are less sterically favoured(0.2679 kcal mol-1 more than the exo dimer). The difference in torsion of the exo and endo dicyclopentadiene is the largest compare to other differences(1.8555 kcal mol-1), which is majorly contributed by unfavourable interaction of the out pointing part of the bicyclic system and two upwards pointing hydrogens on the same face presented in the endo product.
Hydrogenation of Cyclopentadiene Dimer
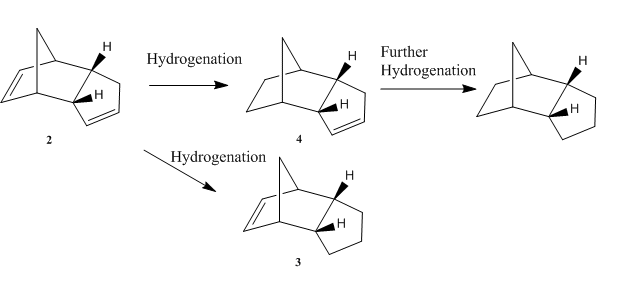
Hydrogenation of the endo-dicyclopentadiene result in either dihydro derivatives 3 or 4 and with further hydrogenation, can form the tetrahydro derivative. The hydrogenation is driven by relief of ring strain(sp2 to sp3).
MM2 calculations are performed in order to investigate the relative stability of 3 and 4.
Results present as follow:
| Energy Terms / kcal mol-1 | Dihydro Derivatives 3 Jmol | Dihydro Derivatives 4 Jmol | Difference in energy (Exo-Endo) |
| Stretch | 1.2771 | 1.0965 | 0.1806 |
| Bend | 19.8664 | 14.5231 | 5.3433 |
| Stretch-Bend | -0.8346 | -0.5494 | -0.2852 |
| Torsion | 10.8068 | 12.4977 | -1.6909 |
| Non-1,4 Vdw | -1.2257 | -1.0693 | -0.1564 |
| 1,4 Vdw | 5.6330 | 4.5128 | 1.1202 |
| Dipole/ Dipole | 0.1621 | 0.1406 | 0.0215 |
| Total Energy | 35.6850 | 31.1520 | 4.533 |
While similar values of 'stretch','stretch-bend','Non-1,4 Vdw' and 'dipole/dipole' energies were obtained from the calculation, the energy values of 'Bend'(5.3433 kcal mol-1) and 'Van der Waals (VdW)'(1.1202 kcal mol-1) energies of dihydro derivative 4 are considerably lower and the 'torsion'(1.6909 kcal mol-1) energy of dihydro derivative 4 are higher. This could be rationalised by steric interactions and strain. Double bond in the norbornene is slightly longer[3], therefore weaker orbital overlap and hence more easily hydrogenated and produce dihydro derivative 4.
Dihydro derivative 4 is of a lower energy compared to dihydro derivative 3(4.533 kcal mol-1). Therefore, dihydro derivative 4 is more stable, which means it's the thermodynamic product. The kinetic product cannot be found using the given data, further more investigation(e.g. experiment) needed.

Atropisomers are stereoisomers with restricted free rotation about a single bond due to steric hinderance.
The key intermediate in the synthesis of Taxol(9&10)are atropisomers, proposed by Elmore and Paquette[4], where the carbonyl group pointing either up or down due to restricted rotation across the ring.
Molecular mechanic (MM2) force field calculations and MMFF94 (a more sophisticated method ) minimisation are run in order to compare the relative stablility of two atropisomers.
Results present as follow:
| Energy Terms / kcal mol-1 | Taxol Intermediate 9 Chair Jmol | Taxol Intermediate 9 Twist Boat Jmol | Taxol Intermediate 10 Chair Jmol | Taxol Intermediate Twist Boat Jmol |
| Stretch | 2.7030 | 2.9473 | 2.6195 | 2.9143 |
| Bend | 12.6819 | 17.2053 | 11.3319 | 16.8212 |
| Stretch-Bend | 0.4468 | 0.5008 | 0.3435 | 0.4399 |
| Torsion | 22.2395 | 21.2900 | 19.6799 | 20.2225 |
| Non-1,4 Vdw | -1.7339 | -1.4159 | -2.1625 | 0.1441 |
| 1,4 Vdw | 14.2266 | 14.5071 | 12.8724 | 14.3464 |
| Dipole/ Dipole | -1.7021 | -1.7302 | -2.0017 | -1.7239 |
| Total Energy | 48.8618 | 53.3044 | 42.6831 | 53.1646 |
| Energy Terms / kcal mol-1 | Taxol Intermediate 9 Chair Jmol | Taxol Intermediate 9 Twist Boat Jmol | Taxol Intermediate 10 Chair Jmol | Taxol Intermediate Twist Boat Jmol |
| Total Energy | 67.7864 | 76.3018 | 60.5534 | 77.8752 |
For the same structure drawn(i.e. 9 or 10), two sets of different values are found for each method used. The different in results corresponds to different conformation of cyclohexane: chair and twist-boats, which are local minima(overall minimum for chair), hence stable enough as the result of calculation. Although there are two form of twist-boats presents, they are enantiomers, hence, same level of energy. As a result only two sets of different values are found for each method used.
The chair conformation of Taxol intermediates 10 is the lowest in energy, hence, the thermodynamic product. Compare to other forms the chair conformation of 10 are lower in energy in terms of '1,4 Vdw' and 'Dipole/ Dipole'. In general, chair conformation are favoured as compared to the twist boat conformation(around 5 kcal mol-1 for 9 and 11 kcal mol-1 for 10). This difference in energy are majorly contributed by the 'Bend' energies which result from the more steric interations within the molecule. The total energy of the twist boat conformation does not differ by a large amount in the MM2 calculation(only around 0.14 kcal mol-1).
Same trends as stated above are found with the MMFF94 minimisation.
Taxol intermediates 9 & 10 are also good examples of hyperstable alkene[5] (olefins) which refer to constrained trans-cycloalkene with a minimum 8 carbon in bridgehead structures which contributes to extra stability. Hence, less reactive and less susceptible to hydrogenation.
Modelling Using Semi-empirical Molecular Orbital Theory
Regioselective Addition of Dichlorocarbene
Reaction Scheme of 9-Chloro-1,4,5,8-tetrahydro-4a,8a-methanonaphthalene with naphthalene reported by Rzepa.[6]
MM2 force field calculation is done in order to have further investigation of the molecule.
Results present as follow:
| Energy Terms / kcal mol-1 | Stretch | Bend | Stretch-Bend | Torsion | Non-1,4 Vdw | 1,4 VDW | Dipole/Dipole | Total Energy |
| MM2 Minimisation Jmol | 0.6193 | 4.7372 | 0.0401 | 7.6590 | -1.0673 | 5.7938 | 0.1123 | 17.8945 |
| MOPAC PM6 Minimisation Jmol | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 19.74026 |
Two structrues are compared and then distance minimisation was performed.
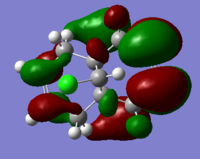
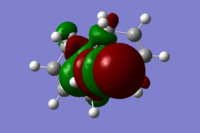
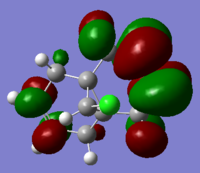
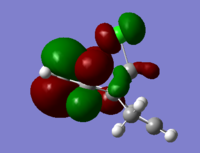
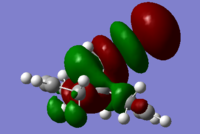
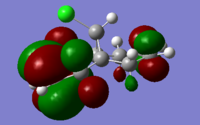
The molecular orbitals (HOMO-1, HOMO, LUMO, LUMO+1, LUMO+2) obtained form PM6 optimization are shown as follow:
The optimisation file is linked to here.
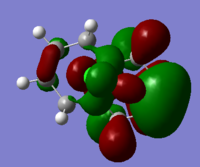
Generally, across the table, less bonding charater, more anti-bonding characters are shown. For HOMO-1, both alkene experience overall bonding interactions. The charge are located on the alkene that's further away from Cl(syn).For HOMO, electron density are mostly located on the C=C that's closer to Cl atom(anti). Therefore, more susceptible to electrophlic attack(dichlorocarbene). The electron density is very widely spreaded in the LUMO orbital, more anti-bonding π*C=C interactions are experienced by the anti alkene as compared to syn. The LUMO+1 MO looks very interesting, with most of the electron density located on the 3 membered ring. For LUMO+2, larger anti-bonding π*C=C interactions is experienced by syn olefin. The syn C=C has a higher frequency than anti C=C. Hence, the syn bond has a higher electron density so will be stronger than the anti C=C and will also be attacked preferentially by an electrophile.
The vibrational spectrum are calculated.
The optimisation file is linked to here
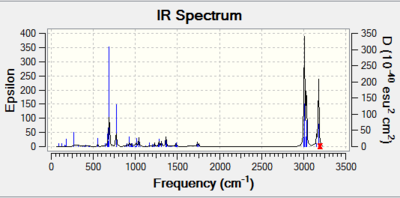
The IR stretches obtained are consistent to literature values.
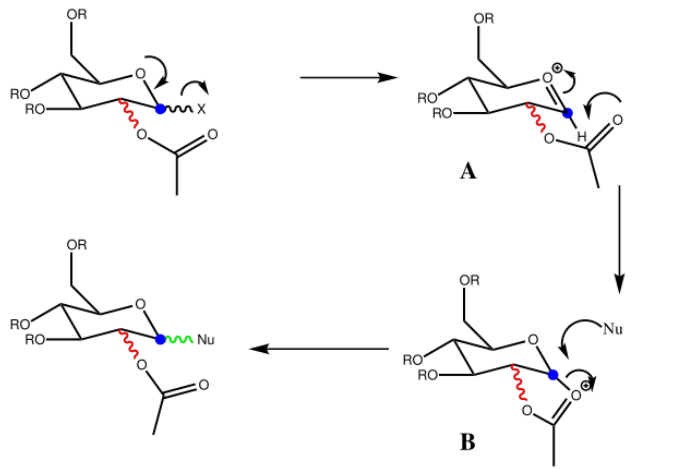
Monosaccharide chemistry and the mechanism of glycosidation
The Mechanism of the reaction is shown as follow(R=Me):
Oxenium cation A has 4 conformations as the OAc group can be either axial(A) or equatorial(E) and the rotation of the single bond within the OAc group can be either pointing upwards(U) or downwards(D).
| Energy Terms / kcal mol-1 | A1 (AD) | A2 (AU) | A3 (ED) | A4 (EU) | B1 (A) | B2 (A') | B3 (E) | 4B (E') |
| Stretch | 2.9125 | 2.7554 | 2.4069 | 3.0850 | 2.7142 | 2.6532 | 1.9105 | 1.8497 |
| Bend | 11.4947 | 11.4669 | 11.16211 | 10.7596 | 17.2682 | 20.2911 | 16.3899 | 14.4048 |
| Stretch-Bend | 1.0641 | 0.9675 | 0.8113 | 0.9680 | 0.7708 | 0.7980 | 0.7717 | 0.6029 |
| Torsion | 1.3676 | 5.0044 | 2.6363 | 3.1946 | 8.6777 | 6.0857 | 7.5632 | 7.6644 |
| Non-1,4 Vdw | 2.2167 | -0.4522 | 0.3397 | 3.4660 | -2.8583 | -2.6475 | -1.8394 | -5.1289 |
| 1,4 Vdw | 18.3248 | 19.4348 | 20.5779 | 19.2417 | 19.6472 | 18.2643 | 17.0208 | 18.5162 |
| Charge/ Dipole | -23.6734 | -32.4103 | -17.9726 | -35.3798 | 3.6003 | -2.5387 | -12.6039 | 2.5495 |
| Dipole/ Dipole | 7.6739 | 5.7030 | 6.1333 | 7.7854 | -1.8142 | -1.3013 | -1.9107 | -0.1652 |
| Total Energy | 21.3158Jmol | 32.8238Jmol | 11.6571Jmol | 13.1205Jmol | 48.0240Jmol | 41.6048Jmol | 27.3021Jmol | 40.2934Jmol |
| Mopac/PM6 | -91.64874Jmol | -77.41071Jmol | -77.51018Jmol | -88.52854Jmol | -66.84383Jmol | -67.00369Jmol | -90.51309Jmol | -90.51317Jmol |
Me groups are used as it is the smallest group that can be used which retains the anomeric effect and in the same time reduce time for calculation. MOPAC/PM6 is a more advanced method of calculation which gives a more accurate result. MM2 values are very different to the MOPAC/PM6 values as MM2 method does not give a very accurate result(calculation based on Vdw). MM2 methods are good with calculation of classical cations, but in this case, O cation is present. Inaccurate results given due to the presence of lone pairs.
Beta-anomer is the dominant product of the due to the neighbouring group effect, which corresponds to the energy values.
Week 2:
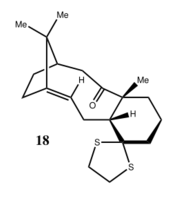
Results present as follow:
| Energy Terms / kcal mol-1 | Stretch | Bend | Stretch-Bend | Torsion | Non-1,4 Vdw | 1,4 VDW | Dipole/Dipole | Total Energy |
| MM2 Minimisation Jmol | 5.3606 | 20.3263 | 0.8744 | 22.5050 | -0.3106 | 17.3746 | -1.7617 | 64.3695 |
The NMR "D-space" file is linked to DOI:10042/22211 (too large,cannot be uploaded)
1H NMR of Taxol Intermediate 18:
13C NMR of Taxol Intermediate 18:
The calculate values for 13C NMR are: 213.025, 150.047, 121.653, 91.1991, 59.3981, 54.405, 49.9417, 49.0343, 46.6216, 41.5174, 39.1675, 34.338, 28.4563, 26.8356, 24.586, 23.2711.This is very similar to literature[9] values.(lit.211.49, 148.72, 120.90, 74.61, 60.53, 51.30, 50.94, 45.53,43.28,40.82, 38.73, 36.78, 35.47, 30.84.30.00, 25.56, 25.35, 22.21, 21.39, 19.83)
The calculate values for 1H NMR are: 6.14703,3.35045,3.31618,3.18734,3.0762, 3.03317, 2.89875, 2.84285, 2.74142, 2.6262, 2.54453, 2.40218, 2.36538, 2.18123,2.13334,1.98819, 1.74833, 1.69709, 1.44999, 1.42475, 1.37768, 1.35973,1.14674, 1.06093, 0.782645.[lit.5.21 (m, 1 H), 3.00-2.70 (m, 6 H), 2.70-2.35(m, 4 H), 2.20-1.70 (m, 6 H), 1.58 (t, J = 5.4 Hz, 1 H), 1.50-1.20 (m,3 H), 1.10 (s, 3 H), 1.07 (s, 3 H), 1.03 (s, 3 H)].
The calculated values for 1H NMR, however, does not correspond to the values that well. The H with the highest calculated chemical shift is 6.14703, whereas in literature, 5.21. This might be caused by interaction with solvents.
Selected Molecule: 2-ethylidene-norbornane (ENBH) E/Z isomers
The reaction[10] proceed as shown:
E/Z-ENBH are formed from acetic acid and can undergo further hydrogenation.
Predicting the 1H and 13C NMR Spectrum of ENBH
The NMR "D-space" file for E-ENBH is linked to DOI:10042/22214 (too large,cannot be uploaded)
The NMR "D-space" file for Z-ENBH is linked to DOI:10042/22215 (too large,cannot be uploaded)
1H NMR of E-ENBH:
13C NMR of E-ENBH:
1H NMR of Z-ENBH:
13C NMR of Z-ENBH:
13C NMR values of E-ENBH:144.159, 111.603, 48.0829, 40.0065, 38.8072,36.9096, 31.6183, 30.4585, 16.6567.(lit.45.2 145.9 35.5 36.5 28.6 29.8 39.1 110.7 13.4)
1H NMR values of E-ENBH:5.7132, 2.69543, 2.40759, 2.31717, 2.04225, 1.79549, 1.72441, 1.70989, 1.592, 1.58642, 1.40575, 1.37886.
13C NMR values of Z-ENBH:143.616, 112.349, 42.759, 40.1223, 38.4838, 31.1401, 30.3445, 17.3252.(lit.39 6 144.9 38.9 36.3 28.4 29.0 38.7 111.5 14.1)
1H NMR values of Z-ENBH:5.47357, 3.09783, ,2.33498, 2.32389, 2.01149, 1.94364, 1.80556, 1.74002, 1.69319, 1.43658, 1.36659.
The calculated 13C NMR mostly corresponds to the literature[11] values, other then the chemical shift for the methyl terminal carbon. E-ENBH: 16.6567(13.4); Z-ENBH: 17.3252(14.1). The large difference compare to other C can be explained as the terminal C are more susceptible to different types of interactions as it is less sterically hindered.
In both isomers, the highest chemical shift in the 1H NMR correspond to the H that is directly linked to the C=C, as it is most deshielded caused by the ring current arise from the C=C. and the second highest chemical shift corresponds to the H that is 1 C away from the C=C(linked to the bridgehead), which also experience a certain degree of deshielding by the ring current from C=C.
Predicting the nJH-H couplings of ENBH
The nJH-H couplings "D-space" file for E-ENBH is linked to DOI:10042/22216 (too large,cannot be uploaded)
The nJH-H couplings "D-space" file for Z-ENBH is linked to DOI:10042/22217 (too large,cannot be uploaded)
Predicting the IR Spectrum of ENBH
IR spectrum of E-ENBH:
| Energy / cm-1 | Form of Vibration | Frequency |
| Alkene C=C |  |
1768.19 |
| Methyl-H |  |
3118.20 |
| Alkene-H |  |
3138.99 |
IR spectrum of Z-ENBH:
| Energy / cm-1 | Form of Vibration | Frequency |
| Alkene C=C |  |
1766.70 |
| Methyl-H |  |
3134.80 |
| Alkene-H |  |
3121.61 |
Predicting the Optical Rotation (OR) and the Electronic Circular Dichroism (CD/UV-Visible) Spectrum of ENBH
The log file for optical rotation of E-ENBH is linked to here
The log file for optical rotation of Z-ENBH is linked to here
The OR for E-ENBH is: [Alpha] ( 5890.0 A) = 103.40 deg. 67878
The log file for Electronic Circular Dichroism of E-ENBH is linked to here
The log file for Electronic Circular Dichroism of Z-ENBH is linked to here
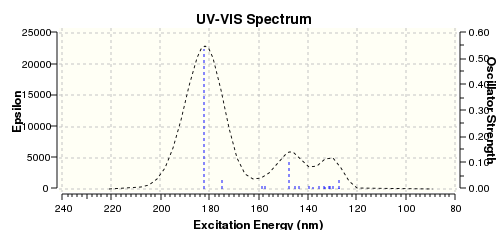
The UV-VIS spectrum oof E-ENBH is calculated:
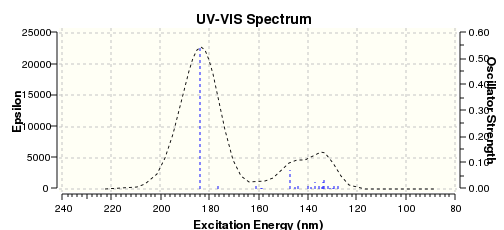
The UV-VIS spectrum oof Z-ENBH is calculated:
Comparing to the UV-VIS spectrum of E-ENBH, Z-ENBH's two peaks at around 140nm are not that obvious, a larger overlap is observed.
Density functional MO calculations for ENBH
The fchk file for MO calculations of E-ENBH is linked to here
The fchk file for MO calculations of Z-ENBH is linked to here
| MOs | E-ENBH HOMO | E-ENBH LUMO | Z-ENBH HOMO | Z-ENBH LUMO |
| PM6 Top View |  |
 |
 |

|
| PM6 Side View | 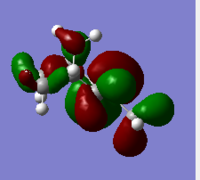 |
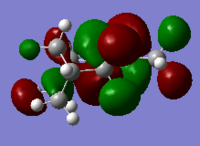 |
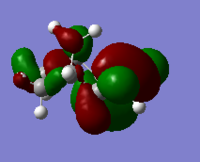 |

|
Generally, for HOMO orbitals, large electron densities are located along the C=C bond, whereas for LUMO, nodal planes are observed across the C=C.
References
1. R. Hoffmann, R. B. Woodward, "Conservation of orbital symmetry", Accounts of Chemical Research, 1968,1, 17-22, DOI:10.1021/ar50001a003
2. J. E. Baldwin, J. Org. Chem., 1966, 31, 244
3. J.J. Zou, X. Zhang, J. Kong, L. Wang, Fuel, 2008, 87, 3655: DOI:10.1016/j.fuel.2008.07.006
4. S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319; DOI:10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-010.1016/S0040-4039(00)92617-0
5. Wilhelm F. Maier, Paul Von Rague Schleyer, J. Am. Chem. Soc., 1981, 103, 1891. DOI: 10.1021/ja00398a003
6. B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447. DOI:10.1039/P299200004477
7. G. Socrates, Infrared and Raman Characteristic Group Frequencies, 3rd Edition, 2001, p. 65.
8. J. Coates, “Interpretation of Infrared Spectra, A Practical Approach", Encyclopedia of Analytical Chemistry, John Wiley & Sons Ltd, Chichester, 2000
9. L. Paquette, N. A. Pegg, D. Toops, G. D. Maynard, R. D. Rogers, J. Am. Chem. Soc.,, 1990, 112, 277-283. DOI:10.1021/ja00157a043
10. R. Winters,†, W. Heinen,†,‡, M. A. L. Verbruggen,§, J. Lugtenburg,†, M. van Duin,‖ and, and H. J. M. de Groot*,†Macromolecules 2002 35(5),1958-1966
11. G. Van der Velden Macromolecules, 1983 16(1),85-89