Rep:Mod:KRMO
Conformational analysis using Molecular Mechanics
The Hydrogenation of Cyclopentadiene Dimer
The exo (product 1) and endo (prduct 2) forms of the cyclopentadiene dimer were created in Avogadro. The geometries of these molecules were then optimised using the MMFF94(s) force field option. The final energies of the molecules were 243.663 kJ/mol and 231.838 kJ/mol for the endo and exo form respectively. jmols of the optimised molecules is given below, with the endo form having a black background and the exo in blue.
endo |
exdo |
The energies of these molecules would seem to suggest that the exo form is preferable, as it is lower in energy. The fact that the endo is specifically formed suggests that the reaction goes kinetically rather than thermodynamically.
The endo form was then then hydrogenated at the two different double bonds in turn to give products 3 and 4. The energies of these molecules were tabulated below.
| Component | Product 3 energy | Product 4 energy |
|---|---|---|
| Stretching | 3.31140 | 2.82307 |
| Bending | 29.85142 | 23.02861 |
| Torsion | -1.47397 | -0.37814 |
| Van der Waals | 13.63730 | 10.63692 |
| Electrostatic | 5.11951 | 5.14702 |
| Total Energy | 50.44566 | 41.25749 |
From the literature, it is seen that product 4 dominates[1]. As product 4 is the lower energy molecule, it can be suggested that the hydrogenation goes thermodynamically rather than kinetically (this is in contrast to the dimerisation).
There is an appreciable difference in bending energy between the two molecules. This could be due to steric interactions between the newly added hydrogens of the hydrogenation of product 3, and the existing, neighbouring hydrogens raising the energy of the molecule.
Taxol, a drug for the treatments of cancer, is synthesised via an intermediate, which is either atropisomer 9 or 10 as in figure 1 below. The objective of this part of the experiment is to determine the relative stability of the two atropisomers.
The cyclohexane fragment of the molecule itself can adopt a number of conformations. The lowest energy conformation of the cycloehexane is the chair conformation and because there are two chair conformations there were two lowest-energy conformations to look for, per atropisomer. The molecules were created in ChemDraw, the exported to Avogadro where they were optimised using the MMFF94(s) force field as before. The final enegies are tabulated below.
| 9 conformer 1 | 9 conformer 2 | 10 conformer 1 | 10 conformer 2 | |
|---|---|---|---|---|
| Final Energy (kcal/mol) | 74.78566 | 67.71628 | 60.03756 | 69.25388 |
The energies of the most stable chair form of each anitropisomer (9 conformer 2 and 10 conformer 1) are tabulated below
| Component | Product 9 conformer 2 | Product 10 conformer 1 |
|---|---|---|
| Stretching | 7.15612 | 7.06079 |
| Bending | 23.7622 | 20.19479 |
| Torsion | 3.46941 | -0.26185 |
| Van der Waals | 33.10094 | 32.24291 |
| Electrostatic | 0.22762 | 0.80162 |
| Total Energy | 67.71628 | 60.03756 |
It is clear from the table that the most stable intermediate is a chair conformation of product 10. The biggest differences in energy occur between the "Bending" and "Torsion". The carbonyl carbon is sp2 hybridised and so would prefer a bond angle of 120° with it's neighbouring atoms. Product 9 has an angle of 118°, whilst 10 has an angle of around 121°. This deviation from the ideal could be causing the energy difference, although it is perhaps too small a deviation to be causing the difference alone.
The alkene reacts slowly because it is a hyperstable olefin. This is an alkene that is less strained than the equivalent alkane[2]. The bridgehead alkene is less strained than the alkane, so when it is hydrogenated, strain increases. This is unfavourable and so proceeds slowly.
Spectroscopic simulation using quantum mechanics
Molecules 17 and 18 are derivatives of the previous products 9 and 10. 1H and 13C spectra were be simulated for 17 and 18 and then compared to existing experimental spectra in the literature.
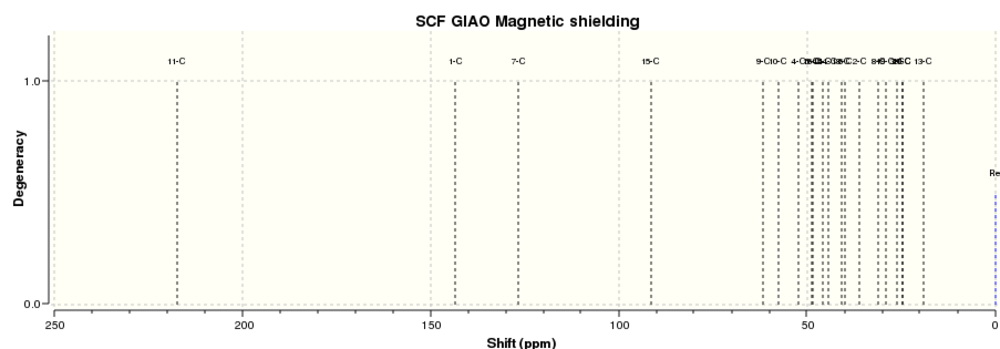
Firstly, the lowest energy chair conformers of product 17 and 18 were created in Avogadro then the geometries optimised using the MMFF94(s) force field option. Once optimised,17 and 18 were analysed to predict the resulting NMRs. The calculation was set to Geometry optimisation, at the theroy level B3LYP using a 6-31G(d,p) basis set. The solvation keyword CRF=(CPCM,Solvent=chloroform) was also used, as well as the keywords FREQ, NMR and EmpiricalDispersion=GD3. The resulting data for the more stable product 18 is given below.
| 1H Literature Value | Computed 1H value | 13C Literature Value | 13C Computed Value |
|---|---|---|---|
| 1.03 (s, 3H) | 0.72 (1H) | 19.83 | 19.23 |
| 1.07 (s, 3H) | 1.01 (2H) | 21.39 | 24.67 |
| 1.10 (s, 3H) | 1.12 (1H) | 22.21 | 24.77 |
| 1.50-1.20 (m, 3H) | 1.24 (1H) | 25.35 | 26.21 |
| 1.58 (t, J = 5.4Hz) | 1.40 (1H) | 25.56 | 29.15 |
| 2.20-1.70 (m, 6H) | 1.54 (2H) | 30.00 | 31.29 |
| 2.70-2.35 (m, 4H) | 1.61 (1H) | 30.84 | 36.14 |
| 3.00-2.70 (m, 6H) | 1.70 (1H) | 35.47 | 40.10 |
| 5.21 (m, 1H) | 1.88 (5H) | 36.78 | 41.00 |
| 2.00 (1H) | 38.73 | 44.38 | |
| 2.15 (2H) | 40.82 | 45.74 | |
| 2.41 (2H) | 43.28 | 48.49 | |
| 2.56 (1H) | 45.53 | 48.85 | |
| 2.66 (2H) | 50.94 | 52.35 | |
| 2.99 (1H) | 51.30 | 57.59 | |
| 3.07 (2H) | 60.53 | 61.62 | |
| 3.14 (1H) | 74.61 | 91.39 | |
| 3.22 (1H) | 120.90 | 126.84 | |
| 4.90 (1H) | 148.72 | 143.64 | |
| 211.49 | 217.25 |
(Literature 1H NMR: 300MHz, C6D6 13C NMR: 75MHz, C6D6)
The first thing noticed was the number of peaks. The 13C NMR spectra have the same number of peaks, but the 1H spectra have differing numbers of peaks. This could be because GaussView treats different peaks in one multiplet as separate, differing environments, rather than just a multiplet and so overestimates the number of environments. An example of this is the literature value recorded at 1.2-1.5 ppm, which is ascribed as multiple different environments by GaussView.
The absolute values of 13C shifts were quite similar, most being within just a few ppm. There is however one exception, which id reported in the literature as 91.39 ppm which is substantially different to the computed value. This corresponds to the C in the cyclohexane ring which is bonded to the two S atoms. The difference in shift could come from the heavy S atoms causing a spin-orbital coupling error which wasn't factored in in the computed model.
1H NMR shifts are similar (within a few fractions of a ppm), however it is difficult to see exactly which literature peak matches which computed peak because of the multiplet-splitting effect mentioned previously. The small difference may be due to the sample used in the literature may not have been composed entirely of the chair conformation. There could be a difference even between the two different chair confirmations in terms of NMR output, but how much difference this actually makes, if any, is unknown. The difference could also be due to the fact that the computed and literautre experiments use differing solvents.
The NMR spectra are given below.

Thermochemical analysis
The relative energies of two molecules were extracted from previous calculations and are shown below.

As can be seen from the data above, product 18 was marginally more stable; as expected.
Analysis of the properties of the synthesised alkene epoxides
NMR Analysis
Shi Catalyst
The Shi catalyst reacts selectively to give the trans epoxide when reacted with a trans alkene. An intermediate in the formation of the catalyst is product 21 which is given below. The bond lengths corresponding to those of the diagram are also tabulated below.
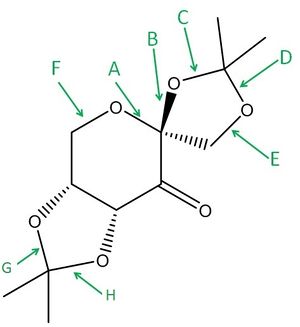
Pentahelicene |
| C-O bond label | Bond length (Å) |
|---|---|
| A | 1.408 |
| B | 1.401 |
| C | 1.443 |
| D | 1.413 |
| E | 1.345 |
| F | 1.404 |
| G | 1.409 |
| H | 1.439 |
As expected, there are longer bonds (C,D,G and H) in the 5-membered rings which contain the anomeric centres. This is because there is good overlap between a high energy lone pair on the O attached to the anomeric centre and the low energy σ* C-O orbital. This puts increaed electron density in the antibonding orbital, weakening and lengthening the C-O bond. This is particularly prominent because the O lone pair is antiperiplanar to the C-O bond.
Not all C-O bonds are weakened. If the overlap between the O lone pair and the σ* C-O orbital is too poor, the bond won't be weakened. This occurs when the arrangement is not close enough to the antiperiplanar ideal.
It might be expected that C&D, and G&H would be very similar lengths, but they are not. This could be to do with the 5-membered ring being not exactly planar, and so the bond orbital overlaps may be different.
The bulky tert-butyl groups cause great steric hindrance for a cis-alkene approaching the dioxyrane oxygens, which makes the catalyst selective for trans-anlkenes.
Jacobson Catalyst
The Jacobson Catalyst asymmetrically epoxidises a number of cis alkenes. Product 23 is the stable pre-catalyst and can be found here. The hydrogens of the two adjacent t-butyl groups on the rings are around 0.24 nm away from each other. This closeness suggests an attractive Van der Waals interaction is occurring. The bulky t-butyl groups on the rings hinder an approach from the front and the sides of the molecule, causing the approaching alkene to attack from on top of molecule.
The calculated NMR properties of styrene and trans-β-Methylstyrene
The two alkenes chosen for epoxidation were styrene and trans-β-Methylstyrene. This gave 4 different epoxides. The molecules were first created with ChemBio 3D, then copied to Avogadro where the energies were minimised. The HPC was then used to compute an NMR; one spectra per original alkene. The results are shown below.
| 1H Literature Value | Computed 1H value | 13C Literature Value | 13C Computed Value |
|---|---|---|---|
| 2.75 (dd, J = 2.8, 5.6, 1H) | 2.53 (1H) | 51.0 | 53.46 |
| 3.08 (dd, J = 4.1, 5.6, 1H) | 3.11 (1H) | 51.1 | 54.05 |
| 3.80 (dd, J = 2.8, 4.1, 1H) | 3.66 (1H) | 125.3 | 118.27 |
| 7.24–7.33 (m, 5H) | 7.30 (1H) | 128 | 122.96 |
| 7.49 (4H) | 128.2 | 123.42 | |
| 137.5 | 124.25 | ||
| 135.13 |
(The literature source[3] of the data 1H NMR 400MHz, CDCl3, 13C 100 MHz, CDCl3)
Broadly, it can be seen that the data are very similar. The values for the 1H NMR are mostly within a fraction of a ppm. For the 13C NMR, the values are no more than 5ppm away from the literature.
The one extra peak seen in the computed data comes from 2 atoms in the same environment being separated into two when interpreted. The closeness of the literature to the computed data suggests the right molecule was created.
Below is the data for the trans-β-methylstyrene oxide molecule.
| 1H Literature Value | Computed 1H value | 13C Literature Value | 13C Computed Value |
|---|---|---|---|
| 1.46 (d, J = 5.1 Hz, 3H) | 0.77 (2H) | 18.0 | 13.07 |
| 3.04 (qd, J = 5.1, 2.1 Hz, 1H) | 1.44 (1H) | 59.0 | 57.66 |
| 3.58 (d, J = 2.2 Hz, 1H) | 3.28 (1H) | 59.5 | 58.99 |
| 7.24–7.33 (m, 5H) | 3.99 (1H) | 125.5 | 121.36 |
| 7.39 (1H) | 128.0 | 122.44 | |
| 7.46 (1H) | 128.4 | 122.71 | |
| 7.53 (3H) | 137.9 | 123.08 | |
| 132.81 |
(Literature sources: 1H NMR[4] 400MHz, CDCl3, 13C[5] 100 MHz, CDCl3)
Again, it can be seen that the data are very similar. The values for the 1H NMR are mostly within a fraction of a ppm (apart from the first value). For the 13C NMR, the values are no more than 5ppm away from the literature.
The extra peaks seen in the computed data comes from 2 atoms in the same environment being separated into two when interpreted. The closeness of the literature to the computed data suggests the right molecule was created.
Assigning the absolute configuration of the product
The chiroptical properties of the two epoxidisied, previously optimised products were investigated using Gaussian and the HPC. Wavelengths of light at 589nm were modelled to predict optical rotation. The results are shown below.
| Styrene oxide computed value | Styrene oxide literature value[6] | TBMS oxide computed value | TBMS oxide literature value[7] |
|---|---|---|---|
| -30.08 | -33 | -61.46 | -54.5 |
The styrene oxide that was calculated in this case seems to be the (S) enantiomer and is quite similar to the literature value. The (R) enantiomer has the opposite value according to the literature as expected[8].
The TBMS calculated value too is close to the literature value and suggests that the TBMS oxide formed is the (S,S) enantiomer. The literature values vary wildly however. A cursory glance reveals a range of values suggests between +100 and -100, so it is not known how accurate the recorded value really is.
Estimating the enantiomeric ratio using the calculated transition states
It is possible to guess what kind of mixture of enantiomers will be prodcued by a reaction by analysing which transition state has the lowest energy. In this part, the transition states of the R and S enantiomers for styrene oxide with the previously mentioned Shi catalyst, and the R,R and S,S enantiomers for trans-β-methylstyrene oxide with the Jacobson catalyst were computed. Using the calculated difference in Gibbs Free Energies of the transition states, a ratio of enantiomers can be estimated. The data is tabulated below.
| Difference in Gibbs free energy between R and S enantiomers (J/mol) | Rate constant of R to S transition |
|---|---|
| -1205.10459 | 1.62641 |
The rate constant of 1.62641 implies that the S enantiomer is slightly favoured as the transition state and will produce a final epoxide enantiomer mixture that is 62% S to 38% R.
| Difference in Gibbs free energy between SS and RR enantiomers (J/mol) | Rate constant of SS to RR transition |
|---|---|
| 27478.4851 | 0.00001525998 |
This extremely low rate constant implies that the reaction almost specifically goes to the SS enantiomer. The final mixture is over 99.99% of the S enantiomer.
The rate constant of 1.62641 implies that the S enantiomer is slightly favoured as the transition state and will produce a final epoxide enantiomer mixture that is 62% S to 38% R.
Investigating the non-covalent interactions in the reaction transition state (NCI)
The 4 different forms of the transition state with the Shi catalyst of the trans-beta-methyl styrene (TBMS) series were analysed to find the one with the lowest energy. This was then analysed in GaussView to fine the non-covalent interactions occuring (as shown below).
Orbital |
As can be seen, there are many NCIs. There is a weakly-bonding interaction between the hydrogen on the attached alkene group and a hydrogen in the ring. There is a ring of electron density that occurs between the alkene of the TBMS and a dioxyraine oxygen from the Shi catalyst and shows the bond forming in the transition state.
There is a large spread of green, mildly-attractive interaction between the termindal methyl of the TBMS and the dioxolane fragment of the catalyst. An attractive force alos occurs between the other dioxolane ring and the benzene end of the TBMS.
Inside the dioxolane rings themselves are highly repulsive forces, probably to the lone pairs of the two oxygen atoms. Mildy replulsive forces are also found within the 6-membered ring in the catalyst, possibly again due to lone pair repulsion.
Investigating the Electronic topology (QTAIM) in the active-site of the reaction transition state
The transition state of the reaction of the Shi catalyst and the 4th in the (S,S) series of trans-beta-methyl styrene was subjected to QUTAIM analysis in Avogadro 2. The image below is the result of that analysis.
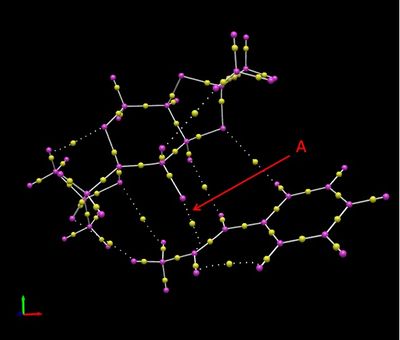
The image shows where the electron density is expected. The centre of electron density between bonds is shown by a yellow bead. Prehaps the most important interaction shown is that labelled, 'A' where the bond-making process occurs. This interaction happens between a dioxyrane oxygen and the TBMS alkene and is not covalent. Other non-covalent interactions displayed include H-bonding like interactions between hydrogen atoms in the TBMS and the dioxolane oxygens from the catalyst.
A new candidate
A possible new candidate is α-pinene, which can be oxidised using the Jacobson catalyst[9] into α-pinene oxide.
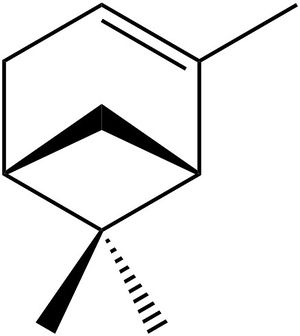
It has an Optical Rotary Power of -34° (589nm)[10], is synthetically availble and is purchasable from Sigma-Aldrich in an optically pure form[11].
References
- ↑ D. Skala and J. Hanika, Petroleum and Coal, 2003, 45, 105–108
- ↑ A. McEwen and P. Schleyer, Journal of the American Chemical …, 1986, 3951–3960.
- ↑ C. Wiles, M. J. Hammond, and P. Watts, Beilstein journal of organic chemistry, 2009, 5, 27. DOI:10.3762/bjoc.5.27
- ↑ D. Romney and S. Miller, Organic Letters, 2012, 1–35.DOI:10.1021/ol3000712
- ↑ L. Ji, Y. Wang, C. Qian, and X. Chen, Synthetic Communications, 2013, 3, 5–9.DOI:10.1080/00397911.2012.699578
- ↑ [1] Accessed 20/11/13
- ↑ H. Toda, R. Imae, and N. Itoh, Tetrahedron: Asymmetry, 2012, 23, 1542–1549. DOI:10.1016/j.tetasy.2012.09.017
- ↑ http://www.sigmaaldrich.com/catalog/product/aldrich/540099?lang=en®ion=GB
- ↑ C. Schuster, E. Möllmann, A. Tompos, and W. Hölderich, Catalysis letters, 2001, 74, 69–75. DOI:10.1023/A:1016639131534
- ↑ Tolstikov et al., Journal of Organic Chemistry USSR (English Translation), 1972 , vol. 8, p. 1204,1207
- ↑ [2] accessed 20/11/2013
