Rep:Mod:KL
Using Gaussian to optimise molecules
NH3 Molecule
Properties

The NH3 molecule on the left was optimised using a B3LYP method and a 6-31G(d,p) basis set with the following steps:
-
Step 1: In the first optimisation step, the N-H bond lengths are 1.3 Angstrom which is too far apart for a covalent bond to form.
-
Step 2: As the optimisation occurs the hydrogen atoms are pulled closer to the Nitrogen atom in the centre.
-
Step 3: The hydrogen atoms now experience an attractive force from the Nitrogen atom and are close enough to form covalent bonds.
-
Step 4: The N-H bonds angle changes from 101.721 to 104.780 to reduce strain in the molecule.
-
Step 5: The bond angle changes again from 104.780 to 105.749.
-
Step 6 of the optimisation process.
-
Step 7: The optimised NH3 molecule.
| Molecule Name | Ammonia (NH3) |
| Calculation Method | RB3LYP |
| Basis set | 6-31G(d,p) |
| E(RB3LYP) | -56.5577687 a.u |
| Point Group | C3V |
| Optimised N-H bond length | 1.01798Å |
| Optimised N-H bond angle | 105.741° |
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
Vibrational modes

NHmolecule |
The number of vibrational modes expected using the 3N-6 rule is 6.
Referring to the table of vibrational frequencies, modes 2 and 3 are degenerate with eachother and so are modes 5 and 6.
Modes 1,2 and 3 are bending modes and modes 4,5 and 6 are stretching modes.
Mode 4 is a highly symmetric mode.
The umbrella mode is mode 1.
There would be 4 bands in the spectrum due to the degeneracy of modes 2 and 3 and modes 5 and 6. This leaves 4 distinct vibrational frequencies at which ammonia absorbs electromagnetic radiation.
Charge Distributions

I would expect there to be a negative charge on the nitrogen atom and positive charges on the hydrogen atoms as nitrogen is the more electronegative of the two.
Reactivity
Nitrogen

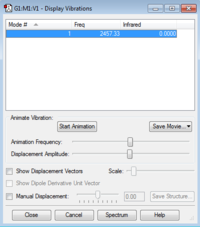
| Molecule Name | Nitrogen (N2) |
| Calculation Method | RB3LYP |
| Basis set | 6-31G(d,p) |
| E(RB3LYP) | -109.52359111 a.u |
| Point Group | D*H |
| Optimised N-N bond length | 1.10550Å |
| Optimised N-H bond angle | 180° |
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
N molecule |
Hydrogen

| Molecule Name | Hydrogen (H2) |
| Calculation Method | RB3LYP |
| Basis set | 6-31G(d,p) |
| E(RB3LYP) | -1.17853936 a.u |
| Point Group | D*H |
| Optimised N-N bond length | 0.74279Å |
| Optimised N-H bond angle | 180° |
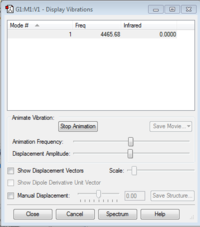
Hmolecule |
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
| E(NH3) | -56.5577687 a.u |
| 2*E(NH3) | =-113.1155374 a.u |
| E(N2) | -109.52359111 a.u |
| E(H2) | -1.17853936 a.u |
| 3*E(H2) | -3.53561808 a.u |
| ΔE=2*E(NH3)-[E(N2)+3*E(H2)] | -0.05579070 a.u |
| ΔE | -146.48kJ/mol |
The energy of the reaction is a negative value indicating that the gaseous product, ammonia, is the more stable than the gaseous reactants.
N2 Molecular Orbitals
-
Molecular Orbital number 1
-
Molecular Orbital number 2
-
Molecular Orbital number 3
-
Molecular Orbital number 4
-
Molecular Orbital number 5
ClF3
Properties

| Molecule Name | Chlorine Trifluoride (ClF3) |
| Calculation Method | RB3LYP |
| Basis set | 6-31G(d,p) |
| E(RB3LYP) | -759.46531682 a.u |
| Point Group | C2V |
| Optimised Cl-F bond length(short bond) | 1.65143 Å |
| Optimised Cl-F bond length (long) | 1.72863 Å |
| Optimised F-Cl-F bond angle (small angle) | 87.140° |
| Optimised F-Cl-F bond angle (large angle) | 174.281° |
Item Value Threshold Converged? Maximum Force 0.000050 0.000450 YES RMS Force 0.000028 0.000300 YES Maximum Displacement 0.000204 0.001800 YES RMS Displacement 0.000134 0.001200 YES
Vibrational Modes
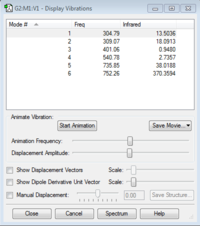
The number of vibrational modes expected using the 3N-6 rule is 6. Referring to the table of vibrational frequencies,none of the modes are degenerate and in the IR spectrum I would expect to find 4 bands as two would overlap being very similar in frequency (modes 1 and 2).
Hmolecule |

Molecular Orbitals
As the 3 fluorine atoms together have 21 electrons which is a higher number than the chlorine which has only 17, the d orbitals in the chlorine are able to be occupied. This is because when the atomic orbitals combine, the resulting bonding molecular orbitals will be lower in energy than some of the antibonding orbitals.
-
Molecular Orbital number 1 is the HOMO in the molecule and is a majority antibonding orbital made up from the combination of the 3px orbital and the 2px orbitals from the fluorines.
-
Molecular Orbital number 2 is a majority antibonding orbital made from the combination of the p orbitals on the fluorines and the 3p orbital on the chlorine.
-
Molecular Orbital number 3 is made from the 3dx2-y2 and the 2p orbitals on the fluorines. It is also a majority antibonding orbital.
-
Molecular Orbital number 4 is made up from the combination of the 3dxy orbital on the chlorine and the 2px from 2 of the fluorines. It is a majority antibonding orbital.
-
Molecular Orbital number 5 is the combination of the 3dxz orbital and the 2p orbitals on the fluorines. It is a majority bonding orbital.
All the molecular orbitals are occupied.
















