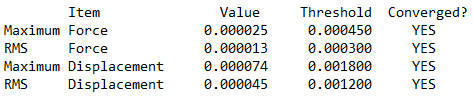Rep:Mod:KIR9876
EX3 Section
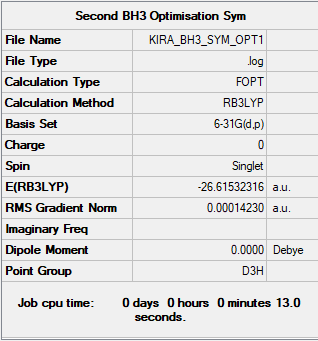
BH3:
Method
B3LYP
Basis set
6-31G(d,p)
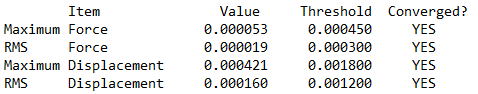
Summary table produced by Gaussview (after optimisation)
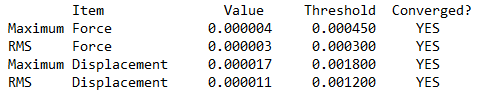
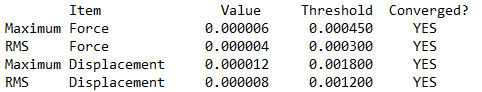
Item table (in pre tags) from the *.log file (from the optimisation job)
Link to the frequency and/or population analysis files
Low frequencies data (in pre tags) from the *.log file (from the frequency job)
Rotatable 3d jmol file/image of the optimised structure
BH3 molecule |
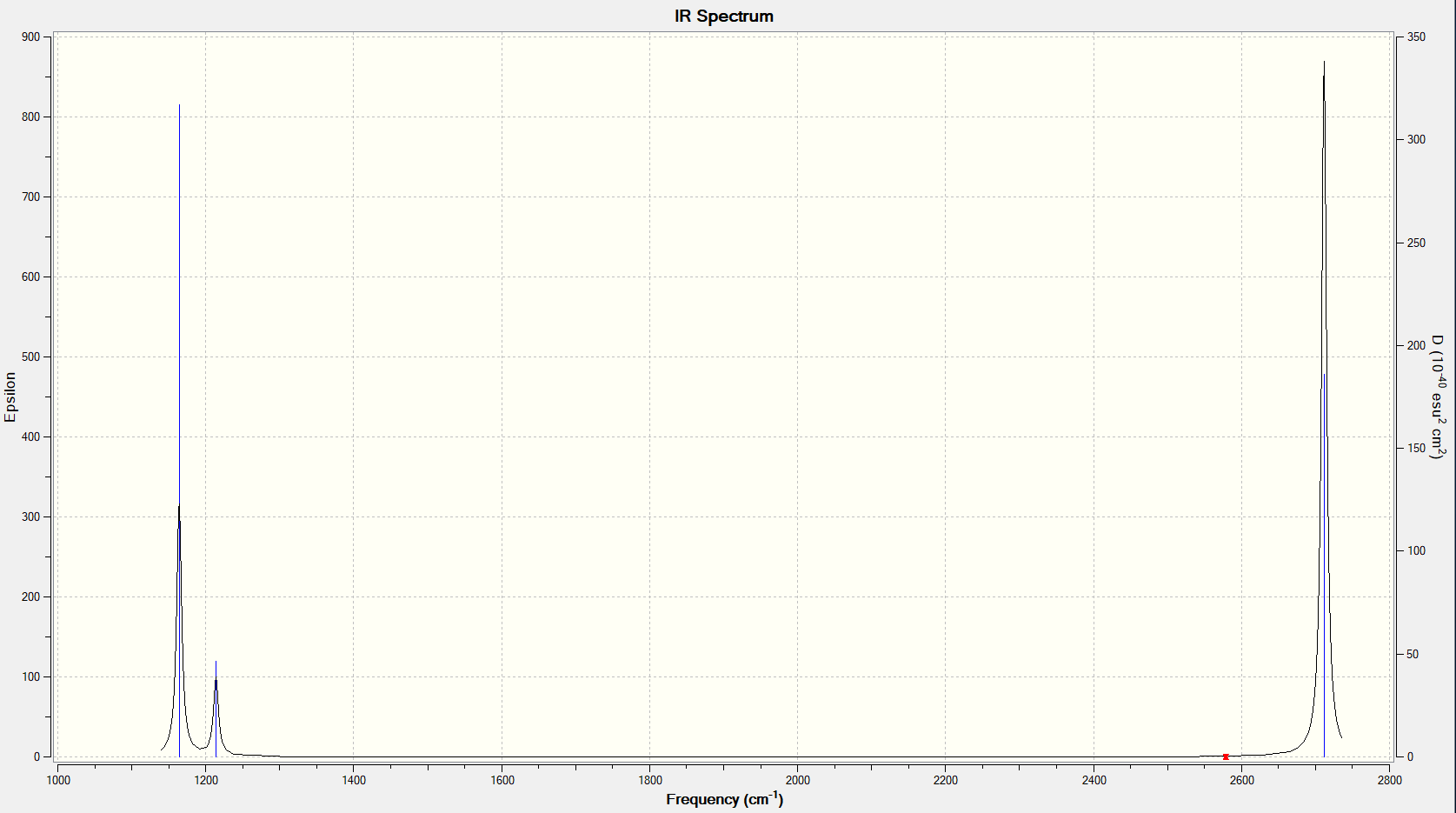
Vibrational Spectrum
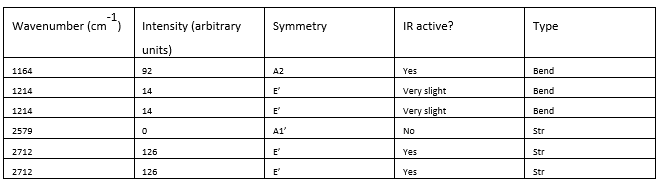
Explain why are there less than six peaks in the spectrum, when there are obviously six vibrations:
There are 2 sets of vibrations that are degenerate (at 1214 cm-1 and 2712 cm-1 respectively) so these peaks overlap, so there are 2 peaks for those 4 different vibrational modes. Out of the 2 remaining vibrational modes, one is IR inactive. For a vibration to be allowed, there has to be a change in dipole moment upon vibrating. For the stretch at 2759 cm-1, the vectors for the vibration cancel out so there is no change in dipole moment, and the intensity of the peak is 0 (that vibration is absent from the spectrum). Therefore there are 3 peaks in the spectrum.
Full information for the vibrational analysis given in the table and a good, clear explanation, great! Just note that a smaller IR table picture would have helped the presentation! Smf115 (talk) 10:00, 30 May 2019 (BST)
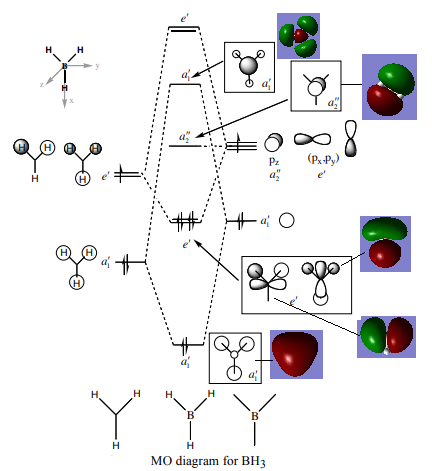
MOs of BH3
MO diagram taken fromː Hunt Research Group, http://www.huntresearchgroup.org.uk/teaching/teaching_comp_lab_year2a/Tut_MO_diagram_BH3.pdf, (accessed 05/19).
Are there any significant differences between the real and LCAO MOs?
The real MOs have the distribution of electron density more spread out among the atoms, showing that electron density is not exclusively localised on the atoms but is more 'smeared' amongst areas of the molecule. For the highest energy LCAO drawn, in the real MO the outer green lobes are larger than the red inside section, whereas in the LCAO drawn the green lobes are smaller than the red inside section. This indicates that perhaps the energy level drawn should be lower than it is as the contributions from the fragments are different from what is expected from the LCAO.
What does this say about the accuracy and usefulness of qualitative MO theory?
Qualitative MO theory is useful in terms of understanding bonding, however it does not explain the bonding within molecules fully. It is not 100% accurate as calculations are needed to determine the exact energy of MOs and a full picture of where the electron density is found. Also qualitative MO theory cannot always accurately predict the energy ordering of the MOs, and it's impossible to be sure until you perform an optimisation of the structure and work out the real MOs. The contributions from the different fragments to the MOs may be different to what LCAO predicts.
Very nice consideration of the similarities and differences between the two sets of MOs. To improve aim to use more technical language, e.g. talk about orbital contributions and which ones specifically than 'red' and 'green'. Additionally, you're sadly missing the LCAOs and calculated MOs for the top two e' MOs! Smf115 (talk) 10:02, 30 May 2019 (BST)
NH3:
Method
B3LYP
Basis set
6-31G(d,p)
Summary table produced by Gaussview (after optimisation)
Item table (in pre tags) from the *.log file (from the optimisation job)
Link to the frequency and/or population analysis files
Low frequencies data (in pre tags) from the *.log file (from the frequency job)
Rotatable 3d jmol file/image of the optimised structure
NH3 molecule |
NH3BH3:
Method
B3LYP
Basis set
6-31G(d,p)
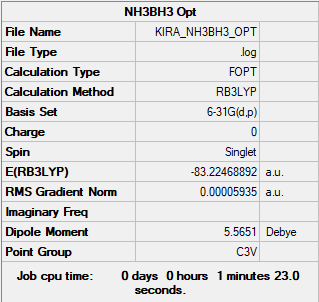
Summary table produced by Gaussview (after optimisation)
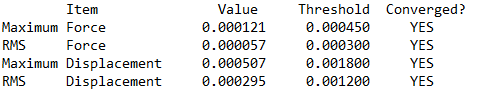
Item table (in pre tags) from the *.log file (from the optimisation job)
Link to the frequency and/or population analysis files
Low frequencies data (in pre tags) from the *.log file (from the frequency job)
Rotatable 3d jmol file/image of the optimised structure
NH3BH3 molecule |
E(NH3)= -56.6 AU = -148603 kJ/mol
E(BH3)= -26.6 AU = -69838 kJ/mol
E(NH3BH3)= -83.3 AU = -218704 kJ/mol
The association energy: ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]= -218704-(-148603-69838)= -263 kJ/mol (energy released on forming the bond)
The dative B-N bond is weak.
Normal B-N bond dissociation energy: +389 kJ/mol (energy we need to put in to break the bond)
So the B-N dative bond is weaker than a normal B-N bond (comparing our dative B-N bond with a normal covalent B-N bond, would need less energy to break our dative bond).
You've done the correct calculation, however, the energies used have been rounded too early resulting in an incorrect answer. The comparison made is good but always include references for literature values! Smf115 (talk) 10:05, 30 May 2019 (BST)
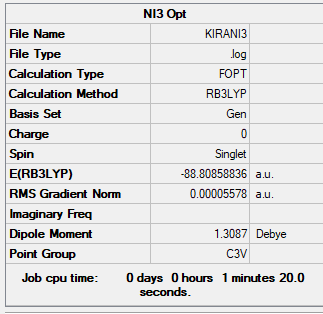
NI3:
Method
B3LYP
Basis set
6-31G(d,p)LANL2DZ
Summary table produced by Gaussview (after optimisation)
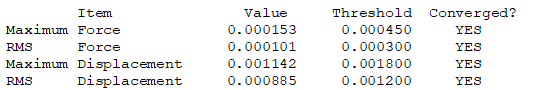
Item table (in pre tags) from the *.log file (from the optimisation job)
Link to the frequency and/or population analysis files
Low frequencies data (in pre tags) from the *.log file (from the frequency job)
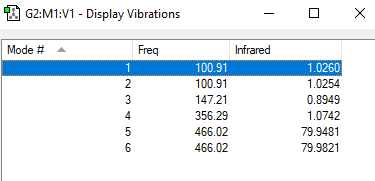
Table of vibrations
There are no negative frequencies.
Rotatable 3d jmol file/image of the optimised structure
NI3 molecule |
What is your optimised N-I distance?
2.184 Å
Project Section - Main Group Halides
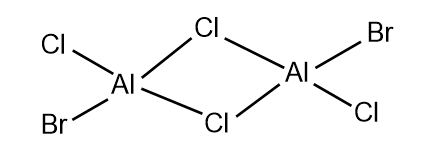
Investigate the conformers and MOs of Al2Cl4Br2.
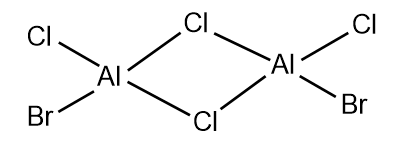
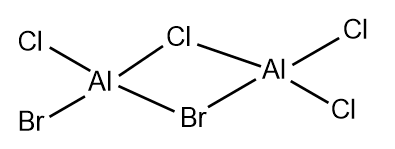
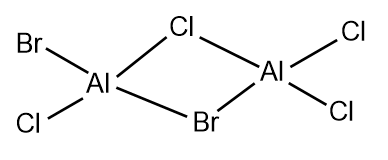
The 5 possible isomers of Al2Cl4Br2 and their symmetry:
Largely correct but you have a repeated isomer which should be C1 symmetry (isomer 3 and 4). You are missing one which has both Br groups as the terminal atoms of the same Al. Smf115 (talk) 23:06, 30 May 2019 (BST)
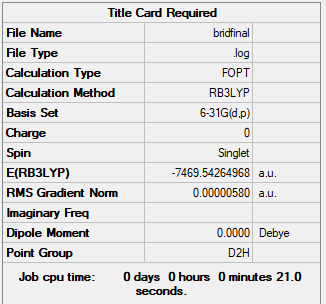
D2h isomer:
Method
B3LYP
Basis set
6-31G(d,p)
Summary table produced by Gaussview (after optimisation)
Item table (in pre tags) from the *.log file (from the optimisation job)
Link to the frequency and/or population analysis files
Low frequencies data (in pre tags) from the *.log file (from the frequency job)
Rotatable 3d jmol file/image of the optimised structure
Al2Cl4Br2 molecule with 2 bridging Br |
C2h isomer:
Method
B3LYP
Basis set
6-31G(d,p)
Summary table produced by Gaussview (after optimisation)
Item table (in pre tags) from the *.log file (from the optimisation job)
Link to the frequency and/or population analysis files
Low frequencies data (in pre tags) from the *.log file (from the frequency job)
Rotatable 3d jmol file/image of the optimised structure
AlCl4Br2 molecule isomer with trans terminal Br and bridging Cl ions |
Energies:
1 AU = 2625.5 kJ/mol
Energy of D2h isomer (isomer with 2 bridging Br ions) = -7469.54265 AU = -19611277 kJ/mol
Energy of C2h isomer (isomer with trans terminal Br and bridging Cl ions) = -7469.53758 AU = - 19611251 kJ/mol
The relative energy of these isomers in kJ/mol
Difference in energy between the D2h isomer and C2h isomer = E(D2h)-E(C2h) = -26 kJ/mol
Discuss the relative stability of these conformers with respect to the bridging ions:-
The D2h isomer with the bridging Br ions is more stable than the C2h isomer with the bridging Cl ions. Br- has a larger ionic radius than Cl- therefore it is easier to accommodate the 2 Al centres around it. The Al-Br bond distance will be longer than the Al-Cl bond distance, so the D2h isomer is lower in energy.
Good justification given as to why the D2h isomer was more stable. However, you haven't implemented the pseudopotential on the Br atoms (LanL2DZ should have been used, as with NI3) resulting in incorrect energies. Smf115 (talk) 23:23, 30 May 2019 (BST)
2AlCl2Br:
Method
B3LYP
Basis set
6-31G(d,p)
Summary table produced by Gaussview (after optimisation)
Item table (in pre tags) from the *.log file (from the optimisation job)
Link to the frequency and/or population analysis files
Low frequencies data (in pre tags) from the *.log file (from the frequency job)
Rotatable 3d jmol file/image of the optimised structure
AlCl2Br molecule |
Determine the dissociation energy for the lowest energy conformer into 2AlCl2Br:
Energy of D2h isomer = -7469.54265 AU = -19611277 kJ/mol
Energy of AlCl2Br = - 3734.74851 AU = -9805583 kJ/mol
Al2Cl4Br2 -> 2AlCl2Br
Dissociation energy= 2* (-9805583) - (-19611277.27) = + 111 kJ/mol
Is the product more or less stable than the isolated monomers?
The isolated monomers of AlCl2Br are less stable than the D2h dimer. Energy needs to be put in (endothermic reaction), in order to dissociate the dimer into 2 monomers. AlCl2Br has a less negative energy the the D2h molecule so the D2h molecule is more stable. It is more stable/energetically favourable to form the dimer than for the species to exist as 2 monomers.
Ok calculation but there has been the same issue with you rounding the energies too early as with all the previous calculations. You have correctly stated the result (dimer more stable) but have also repeated this in different ways, it would have been good instead to see some suggestions as to why the dimer may be more stable. Smf115 (talk) 23:27, 30 May 2019 (BST)
MOs:
These 3 MOs are all valence MOs as they come from the range of the HOMO to the orbital just before the jump in energy to the core orbitals. They are all occupied and come from the lowest energy isomer, which is the D2h isomer.
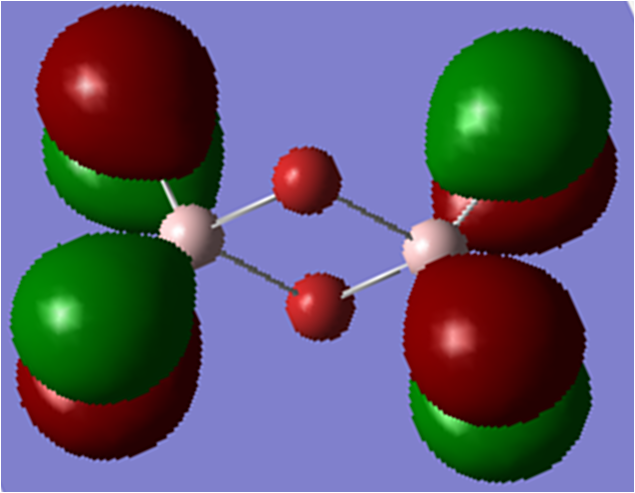
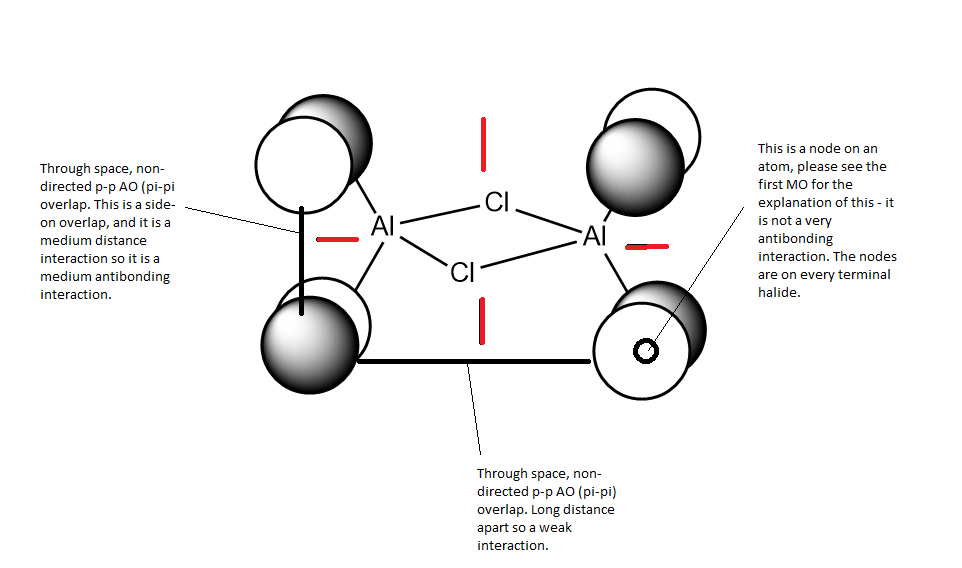
Analysis of first MOː
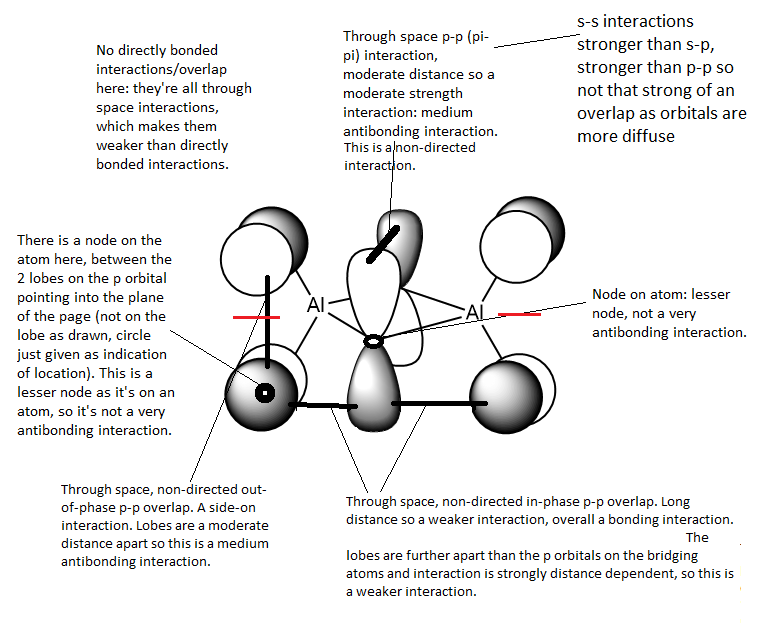
This MO is HOMO-9 (i.e. 9 levels below the HOMO). This is an overall highly bonding MO as there are large areas of in-phase overlap and the antibonding interactions are all relatively weak. In this orbital you can see how both the terminal and bridging atoms contribute to the bonding in the molecule.
There is 1 nodal plane (shown in red) within this molecule which cuts through the nodes on the terminal atoms, the Al atoms and the centre of the bridging unit. It is a medium antibonding interaction as the part through the bridging unit is a nodal plane in the internuclear region (strong antibonding) but the part of the nodal plane cutting though the atoms is only weakly antibonding. The plane doesn't run directly along a bond so it is not as strong of an antibonding interaction.
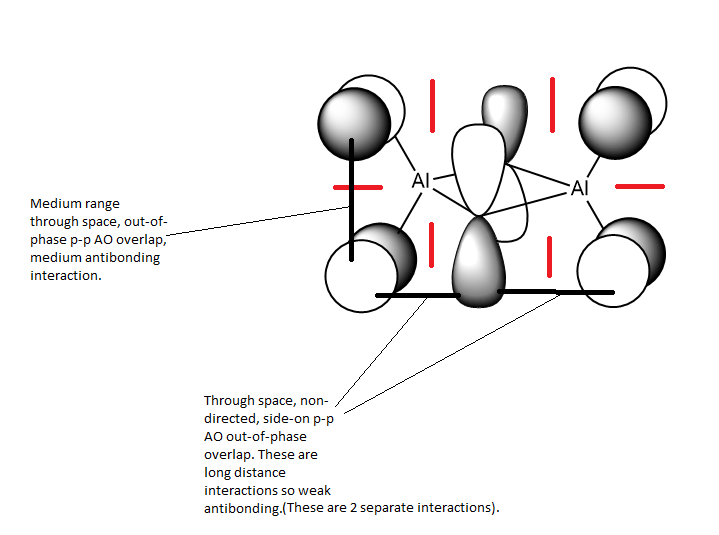
Analysis of second MOː
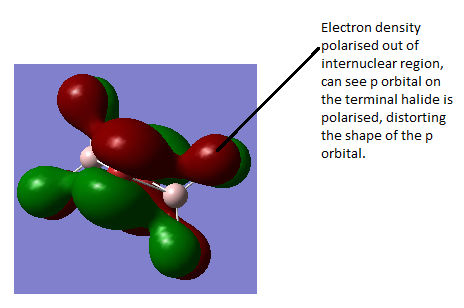
This MO is HOMO-4 (i.e. 4 levels below the HOMO). This is an overall weakly antibonding MO as there is only out of phase interaction in this molecule but only at large distances apart or across a node on an atom or nodal plane. There is no contribution from the bridging atoms in this MO, it only has bonding/orbitals on the terminal atoms. It appears to show a p orbital on each terminal halide atom, with no contribution from the bridging halides. The p orbitals on the terminal halides are polarised away from the Al centre/bridging region as the halide is more electronegative than Al so it attracts the electron density within the orbital more strongly than the Al does.
There are 2 nodal planes (shown in red) within this moleculeː one which cuts through the nodes on the terminal atoms, the Al atoms and the centre of the bridging unit. It is a medium antibonding interaction as the part through the bridging unit is a nodal plane in the internuclear region (strong antibonding) but the part of the nodal plane cutting though the atoms is only weakly antibonding. The plane doesn't run directly along a bond so it is not as strong of an antibonding interaction. The other nodal plane cuts vertically through the MO, this is an antibonding interaction.
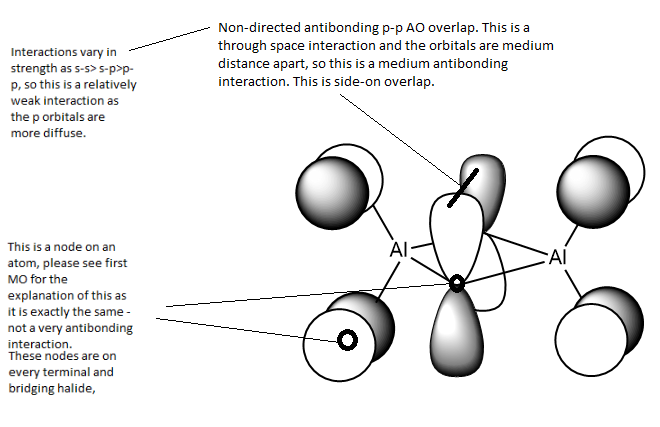
Analysis of third MOː
This MO is HOMO-1 (1 level below the HOMO). This is an overall highly antibonding MO as there are large areas of out-phase overlap any bonding interactions are at such a long distance that they are insignificant. This MO shows antibonding interactions between the terminal and bridging units.
From this MO it appears all the p orbitals contributing are of equal size. There are 3 nodal planes (shown in red) within this moleculeː one which cuts through the nodes on terminal atoms, the Al atoms and the centre of the bridging unit. It is a medium antibonding interaction as the part through the bridging unit is a nodal plane in the internuclear region (strong antibonding) but the part of the nodal plane cutting though the atoms is only weakly antibonding. The plane doesn't run directly along a bond so it is not as strong of an antibonding interaction. The other 2 nodal plane cut vertically through the MO on the Al centres, these are antibonding interactions.
Very thorough MO analysis. You've selected a good range of MOs and constructed the corresponding LCAOs well. The nodal planes and interactions have been described well and used to justify the character of the MO well, however, you repeated a lot of information. A tip for the future is to put the information into the annotations (as you did) and this then doesn't need to be repeated! Also think about using terms such as σ and π interactions to describe some of the overlaps. Smf115 (talk) 23:39, 30 May 2019 (BST)
Overall a good report. Smf115 (talk) 23:39, 30 May 2019 (BST)