Rep:Mod:KFC3008
Borane(BH3)
- BH3 N-H bond length:1.19267 Å
- BH3 N-H-N bond angle:119.99998°
Calculation information
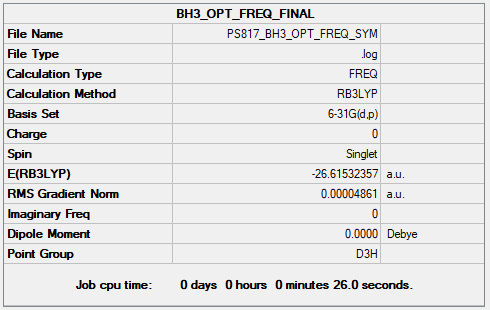
Complete optimisation calculations and generated data can be found here PS817_BH3_OPT_FREQ_SYM.LOG
Item Value Threshold Converged?
Maximum Force 0.000097 0.000450 YES
RMS Force 0.000064 0.000300 YES
Maximum Displacement 0.000386 0.001800 YES
RMS Displacement 0.000252 0.001200 YES
Predicted change in Energy=-5.621702D-08
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1925 -DE/DX = -0.0001 !
! R2 R(1,3) 1.1925 -DE/DX = -0.0001 !
! R3 R(1,4) 1.1925 -DE/DX = -0.0001 !
! A1 A(2,1,3) 120.0 -DE/DX = 0.0 !
! A2 A(2,1,4) 120.0 -DE/DX = 0.0 !
! A3 A(3,1,4) 120.0 -DE/DX = 0.0 !
! D1 D(2,1,4,3) 180.0 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
Vibrational Information
Low frequency lines:
Low frequencies --- -0.3036 -0.1414 -0.0055 35.0909 36.5423 36.5435 Low frequencies --- 1163.3793 1213.4405 1213.4432
BH3 molecule |
Table of vibrational results
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1)1163.38 | 93 | A2" | yes | out-of-plane bend |
| 2)1213.44 | 14 | E' | very slight | bend |
| 3)1213.44 | 14 | E' | very slight | bend |
| 4)2580.94 | 0 | A1' | no | symmetric stretch |
| 5)2713.98 | 126 | E' | yes | asymmetric stretch |
| 6)2713.98 | 126 | E' | yes | asymmetric stretch |
Vibrational spectrum

Despite the fact that there are 6 vibrations that arise from the calculation,
only 3 peaks are present. This is due to two reasons. Firstly some of the vibrations
are double degenerate( E') and thus they only lead to one peak in the spectrum
i.e. vibrations 2&3(1213.44 cm-1) and vibrations 5&6(2713.98 cm-1). Secondly, vibration
4 is not IR active as it is a symmetric stretch that doesn't lead to a change in the
dipole moment of the molecule. Thus it doesn't appear in the spectrum.
Great, clear explanation and full information is given for the vibrational analysis! Smf115 (talk) 22:00, 18 May 2019 (BST)
Molecular orbital calculations
The molecular orbital diagram for BH3 is illustrated below:

T.Hunt, 2018,Problem class 1: the MO diagram of BH3,model answers,
Molecular Orbitals in Inorganic Chemistry, Imperial College London, delivered November 2018.
The calculated Molecular Orbitals are in close agreement with the LCAO's diagrams drawn.
This illustrates the usefulness of LCAO's approach and how MO's can be easily and quickly be
derived from a simple model, with great accuracy without the need of a rigorous calculation.
However , despite the very good agreement, the specific shape of the orbitals cannot be derived
completely using the simple diagram approach. For the exact electronic distribution and any polarizastions
or distortions of the orbitals, a more sophisticated calculation is needed.
NH3BH3
- NH3 calculations
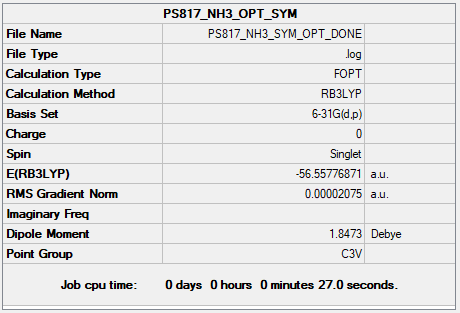
Complete optimisation calculations and generated data can be found here PS817 NH3 SYM OPT DONE.LOG
Item Value Threshold Converged?
Maximum Force 0.000033 0.000450 YES
RMS Force 0.000028 0.000300 YES
Maximum Displacement 0.000215 0.001800 YES
RMS Displacement 0.000153 0.001200 YES
Predicted change in Energy=-1.395785D-08
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.018 -DE/DX = 0.0 !
! R2 R(1,3) 1.018 -DE/DX = 0.0 !
! R3 R(1,4) 1.018 -DE/DX = 0.0 !
! A1 A(2,1,3) 105.7277 -DE/DX = 0.0 !
! A2 A(2,1,4) 105.7277 -DE/DX = 0.0 !
! A3 A(3,1,4) 105.7277 -DE/DX = 0.0 !
! D1 D(2,1,4,3) -111.8308 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
Summary table of results
NH3 molecule |
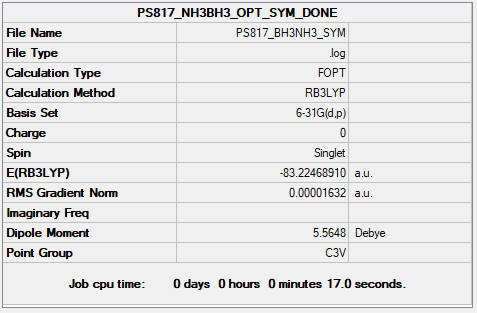
- NH3BH3 calculations Complete optimisation calculations and generated data can be found here PS817 BH3NH3 SYM.LOG
Item Value Threshold Converged?
Maximum Force 0.000070 0.000450 YES
RMS Force 0.000019 0.000300 YES
Maximum Displacement 0.000292 0.001800 YES
RMS Displacement 0.000145 0.001200 YES
Predicted change in Energy=-2.092352D-08
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,8) 1.2097 -DE/DX = 0.0 !
! R2 R(2,8) 1.2097 -DE/DX = 0.0 !
! R3 R(3,8) 1.2097 -DE/DX = 0.0 !
! R4 R(4,7) 1.0184 -DE/DX = 0.0 !
! R5 R(5,7) 1.0184 -DE/DX = 0.0 !
! R6 R(6,7) 1.0184 -DE/DX = 0.0 !
! R7 R(7,8) 1.6674 -DE/DX = 0.0001 !
! A1 A(4,7,5) 107.8787 -DE/DX = 0.0 !
! A2 A(4,7,6) 107.8787 -DE/DX = 0.0 !
! A3 A(4,7,8) 111.0203 -DE/DX = 0.0 !
! A4 A(5,7,6) 107.8787 -DE/DX = 0.0 !
! A5 A(5,7,8) 111.0203 -DE/DX = 0.0 !
! A6 A(6,7,8) 111.0203 -DE/DX = 0.0 !
! A7 A(1,8,2) 113.872 -DE/DX = 0.0 !
! A8 A(1,8,3) 113.872 -DE/DX = 0.0 !
! A9 A(1,8,7) 104.5998 -DE/DX = 0.0 !
! A10 A(2,8,3) 113.872 -DE/DX = 0.0 !
! A11 A(2,8,7) 104.5998 -DE/DX = 0.0 !
! A12 A(3,8,7) 104.5998 -DE/DX = 0.0 !
! D1 D(4,7,8,1) -60.0 -DE/DX = 0.0 !
! D2 D(4,7,8,2) 60.0 -DE/DX = 0.0 !
! D3 D(4,7,8,3) 180.0 -DE/DX = 0.0 !
! D4 D(5,7,8,1) 60.0 -DE/DX = 0.0 !
! D5 D(5,7,8,2) 180.0 -DE/DX = 0.0 !
! D6 D(5,7,8,3) -60.0 -DE/DX = 0.0 !
! D7 D(6,7,8,1) 180.0 -DE/DX = 0.0 !
! D8 D(6,7,8,2) -60.0 -DE/DX = 0.0 !
! D9 D(6,7,8,3) 60.0 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
BH3NH3 molecule |
Ok optimisation information and you've calculated the correct structures. However, you were required to submit the frequency calculations (not optimisation) on the molecules and the structure information (apart from the convergence table) should have also been taken from this calculation too. In general, the frequency calculation is needed to confirm that you are at a minimum energy structure and to check the low frequencies of the molecules too (which are also missing from your report )Smf115 (talk) 21:59, 18 May 2019 (BST)
- ASSOCIATION ENERGY:
- E(NH3)=-56.55776 a.u.
- E(BH3)=-26.61532 a.u.
- E(NH3BH3)=-83.22470 a.u
- ΔE=-83.22468910-(-56.55776871-26.61532357)=-0.05159682 a.u. or -135 kJ/mol
The association energy is negative as expected showing that the dative covelant
bond formation is a favourable process , that ends up lowering the energy of the
system when NH3 and BH3 come together. Furthermore, comparing to the typical Carbon-Carbon
in an alkyl chain with bond energy around 90 kJ/mol and the typical C=O bond of strength 180 kJ/mol,
the dative covalent bond in BH3NH3 of strength 135 kJ/mol can be considered a medium strength bond.
This extra strength, can be attributed to the electronegativity difference of Nitrogen and Boron and
thus the degree ionic nature of the bond. However it is important to note that although a bond has a higher
dissociation energy doesn't mean that it is harder to break. Carbon-Carbon bonds have lower dissociation energy
than C=O bonds but that are much harder to break, due to the lack of polarity.
Correct calculation and nice consideration of the accuracy of your final reported energy. However, I'm not sure where you sourced your comparative bond energies from (always include a reference for literature values!), but they aren't correct and the B-N bond is actually weaker than a C-C bond, meaning that your discussion here isn't right. Smf115 (talk) 21:59, 18 May 2019 (BST)
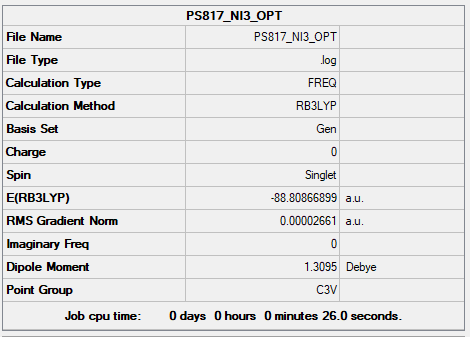
PP and basis sets for NI3
Complete optimisation calculations and generated data can be found here PS817 NI3 OPT.LOG
Item Value Threshold Converged?
Maximum Force 0.000048 0.000450 YES
RMS Force 0.000031 0.000300 YES
Maximum Displacement 0.000333 0.001800 YES
RMS Displacement 0.000250 0.001200 YES
Predicted change in Energy=-3.050626D-08
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 2.1832 -DE/DX = 0.0 !
! R2 R(1,3) 2.1832 -DE/DX = 0.0 !
! R3 R(1,4) 2.1832 -DE/DX = 0.0 !
! A1 A(2,1,3) 110.8881 -DE/DX = 0.0 !
! A2 A(2,1,4) 110.8881 -DE/DX = 0.0 !
! A3 A(3,1,4) 110.8881 -DE/DX = 0.0 !
! D1 D(2,1,4,3) 123.6493 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
Low frequency lines
Low frequencies --- -0.7427 -0.6607 -0.0031 0.0772 0.1683 1.0044 Low frequencies --- 101.3524 101.3530 148.4022
Summary table
Optimised NI3 molecule |
The optimised distance is 2.1832 Å
MINI PROJECT-IONIC LIQUIDS
[N(Me)4]+
- Calculations and Optimisation
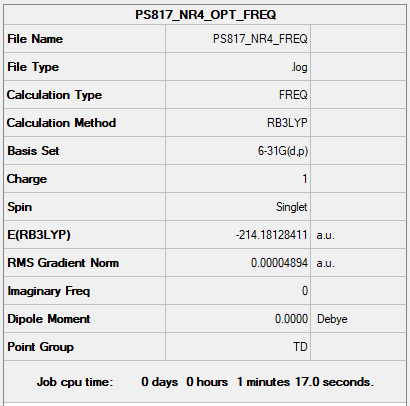
Complete optimisation calculations and generated data can be found here PS817 NR4 FREQ.LOG
Item Value Threshold Converged?
Maximum Force 0.000132 0.000450 YES
RMS Force 0.000049 0.000300 YES
Maximum Displacement 0.000167 0.001800 YES
RMS Displacement 0.000087 0.001200 YES
Predicted change in Energy=-1.011776D-07
Optimization completed.
-- Stationary point found.
Low frequency lines
Low frequencies --- -0.0010 -0.0007 -0.0007 22.6365 22.6365 22.6365 Low frequencies --- 190.5259 293.9048 293.9048
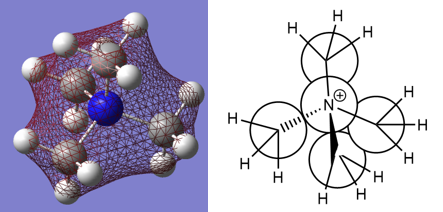
Optimised [N(Me)4]+ molecule |
- Moleclar Orbital Calculations
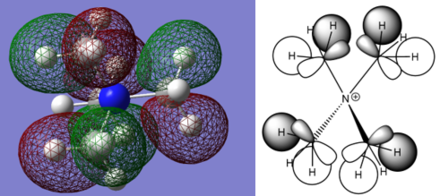
I think you've not fully understood what you were meant to do for this section. For each of the MOs chosen you had to construct the FOs and then use these to form the LCAO representation of the MO. While you have made a nice attempt at analysing the overall charcter of the MOs, the main content is missing for the questions and you haven't labelled the MOs studied either. Smf115 (talk) 22:38, 21 May 2019 (BST)
[P(Me)4]+
- Calculations and Optimisation
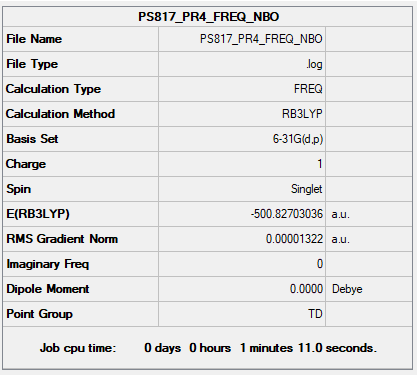
Complete optimisation calculations and generated data can be found here PS817 PR4 FREQ NBO.LOG
Item Value Threshold Converged?
Maximum Force 0.000023 0.000450 YES
RMS Force 0.000013 0.000300 YES
Maximum Displacement 0.000203 0.001800 YES
RMS Displacement 0.000104 0.001200 YES
Predicted change in Energy=-2.738723D-08
Optimization completed.
-- Stationary point found.
Low frequency lines
Low frequencies --- -0.0031 -0.0026 0.0006 24.9812 24.9812 24.9812 Low frequencies --- 160.9839 195.5414 195.5414
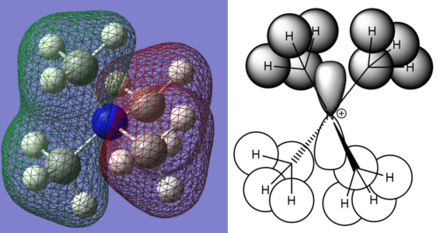
Optimised [P(Me)4]+ molecule |
Charge Distribution Calculations
A full NBO charge analysis was performed with a range of -0.6 to +0.6
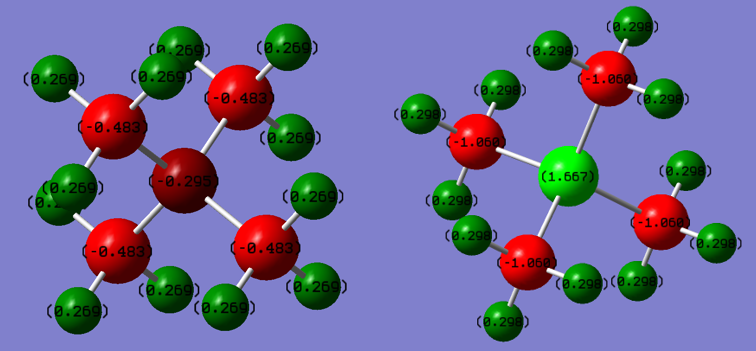
RIGHT: Charge distribution for [P(CH3)4]+.
[N(CH3)4]+ charges(DEBYE):
- Nitrogen: -0.295
- Hydrogen: 0.269
- Carbon: -0.483
[P(CH3)4]+ charges(DEBYE):
- Phosphorous: 1.667
- Hydrogen: 0.298
- Carbon: -1.060
The formal charge on the Nitrogen or Phosphorous is merely a convention, assuming
that electrons in all chemical bonds are shared equally between atoms, regardless of
relative electronegativity. It is also assumed that electrons are localised in the bonds
and thus formal charges in the valence bond model keep track of the electrons around the
atom, in this case N or P. However, as shown in both molecules the charge is delocalised
in the entire molecule .
Specifically in [N(CH3)4]+ the positive charge is found on the electropostive protons, while
the nitrogen, being an electronegative element is negatively charged. This is the opposite of what
of what VSEPR and localisation of bonds predict, exactly because in real molecules, molecular
orbitals that span all over the entire molecule are present, leading to delocalisation of the
charge.
When compared to [N(CH3)4]+ , the charge separation in the C-P bond is greater than in the N-P bond
due to the greater difference in electronegativity between C&P(𐤃elec=0.49) and C&N(𐤃elec=-0.36). Also
the charge on the phosphorous is now positive since phosphorous is more electropostive than nitrogen.
Therefore in this case the charge is greatly found on the phosphorous atom and partly in the hydrogens.
Correct charges calculated and equal colour range used to highlight the charge distributions. Your analysis of the charges using the relative electronegativities is correct but could have been discussed in a bit more depth and included the charges on the H atoms too. Considering other effects, such as symmetry, would also have improved your answer. Smf115 (talk) 21:35, 20 May 2019 (BST)
In terms of discussion the formal charge you are correct in that the electrons are assumed to be localised in the bonds etc. but you don't really explain where the +1 formal charge arises from specifically. Smf115 (talk) 21:35, 20 May 2019 (BST)
An ok attempt at the report which was let down by project section, to improve make sure that you understand the question being asked and if not, then seek help/clarification. Smf115 (talk) 22:39, 21 May 2019 (BST)