Rep:Mod:Jsr3217
BH3
B3LYP/6-21G level
Item Value Threshold Converged? Maximum Force 0.000203 0.000450 YES RMS Force 0.000098 0.000300 YES Maximum Displacement 0.000653 0.001800 YES RMS Displacement 0.000415 0.001200 YES
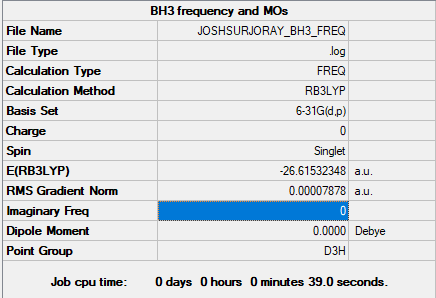
Frequency analysis log file JOSHSURJORAY_BH3_FREQ.log
Low frequencies --- -0.2458 -0.1130 -0.0053 43.9715 45.1306 45.1313 Low frequencies --- 1163.6034 1213.5913 1213.5940
optimised BH3 molecule |
Log Link = https://wiki.ch.ic.ac.uk/wiki/images/5/53/JOSHSURJORAY_BH3_FREQ.LOG
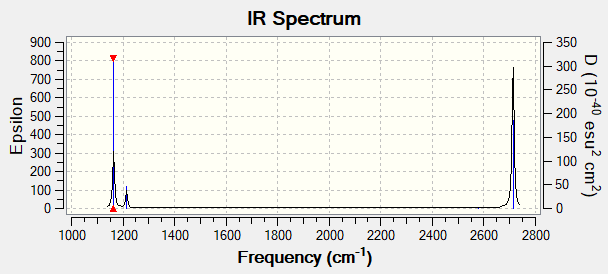
Vibrational Spectrum for BH3
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1164 | 92 | A2" | yes | Out-of-Plane bend |
| 1214 | 14 | E' | Very Slight | Bend |
| 1213 | 14 | E' | Very Slight | bend |
| 2580 | 0 | A' | No | Symmetric Stretch |
| 2713 | 126 | E | no | asymmetric stretch |
| 2713 | 126 | E' | Yes | Asymmetric stretch |
Despite there being 6 vibrations for the molecule, only 3 peaks are shown on the IR spectrum as two of the vibrations are degenerate and are of the same energY. This means they will appear as the same peak on the spectrum. The vibration at 2582 cm-1 does not satisfy the selection rule for IR spectroscopy as that vibration doesn't cause a change in the overall dipole moment and hence is IR silent, not showing up on the spectrum.
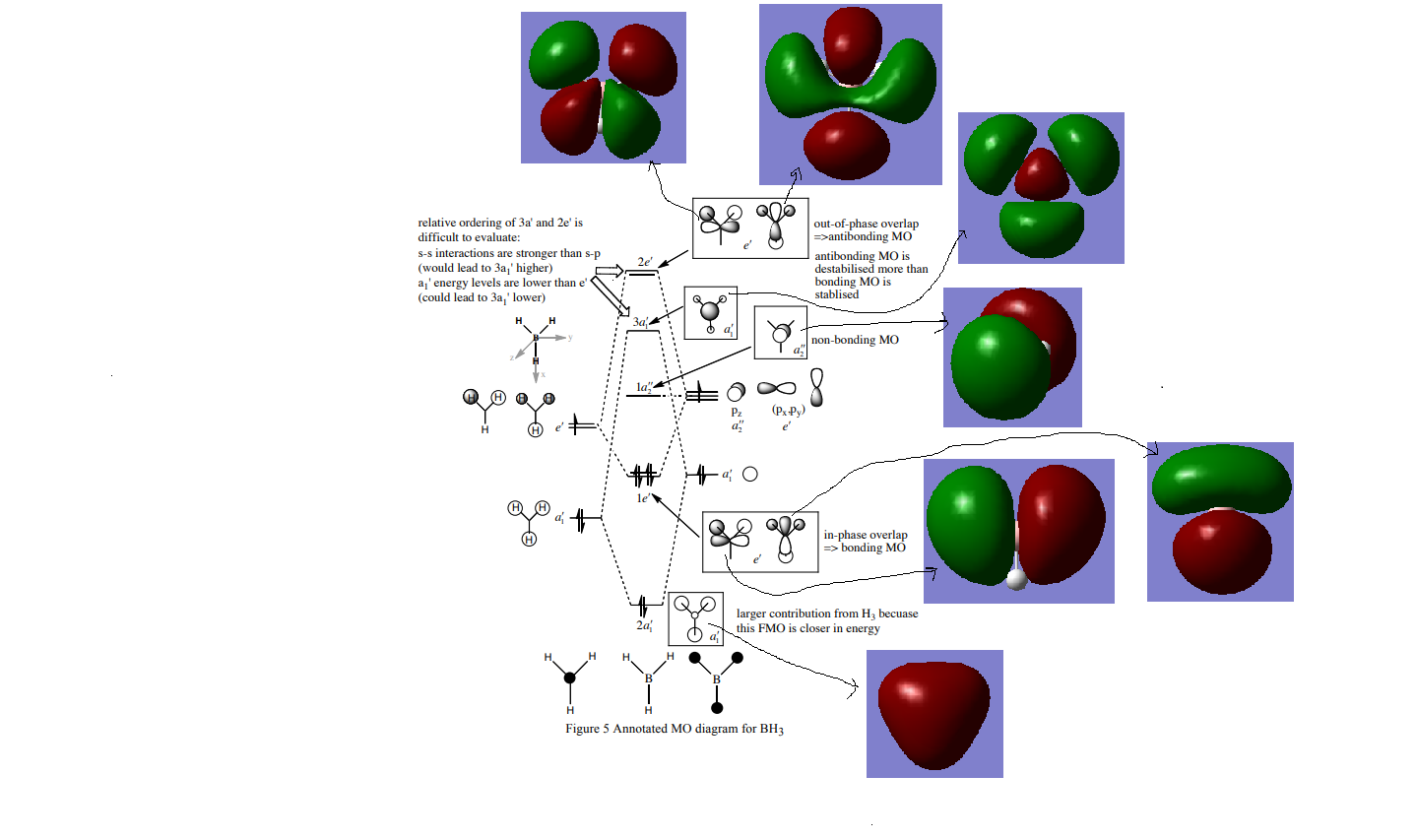
Molecular Orbital Diagram for BH3
MO diagram referenced from: hunt, P, 2018, MO problem class 1, ICL
Good assignment of the calculated MOs to the LCAO MOs and inclusion of them on the diagram. However, you are missing any discussion about the similarities/differences and the usefulness of the LCAO approach. The arrows could have been presented a bit better too using something like chemdraw. Smf115 (talk) 21:52, 16 May 2019 (BST)
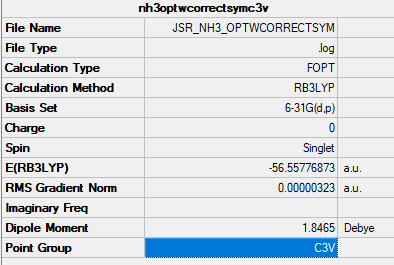
NH3
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000012 0.001800 YES RMS Displacement 0.000008 0.001200 YES
Low frequencies --- -8.5646 -8.5588 -0.0041 0.0455 0.1784 26.4183 Low frequencies --- 1089.7603 1694.1865 1694.1865
optimised NI3 molecule |
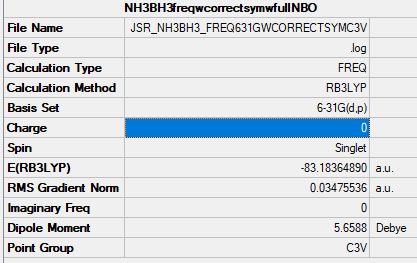
Analysis Log File: JSR_NH3_FREQWCORRECTSYM.LOG
Good inclusion of the optimisation information but the log file link isn't working for the required file. Smf115 (talk) 21:59, 16 May 2019 (BST)
NH3BH3
Item Value Threshold Converged? Maximum Force 0.000121 0.000450 YES RMS Force 0.000057 0.000300 YES Maximum Displacement 0.000507 0.001800 YES RMS Displacement 0.000295 0.001200 YES
Low frequencies --- -0.0576 -0.0501 -0.0075 21.6279 21.6380 40.3364 Low frequencies --- 265.9847 632.3695 640.1234
optimised NH3BH3 molecule |
Association Energy of NH3BH3
E(NH3) = -56.55777 AU
E(BH3) = -26.61532 AU
E(NH3BH3) = -83.18365 AU
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)] = -0.01056 AU = -30 KJ/mol
The enthalpy of formation for this is adduct is negative which implies that the formation of this adduct is favourable. The primary contribution to this negative enthalpy change is the bond energy or strength of the dative bond between N and B as that is the only difference between the reactants and the products of the the reaction. However due to the magnitude of the value we can conclude that the bond strength of this adduct bond is fairly weak as that is the only difference between the reactants and the products of the the reaction although the forward process is favourable.
You've used the correct calculation, presented the energies well and thought about the accuracy of the final reported energy value. However, your NH3BH3 is incorrect, giving an incorrect answer. The error might have been highlighted if you'd compared it to some known bond dissociation enthalpies as required. There hasn't been a log file included for NH3BH3 so I sadly can't see what's gone wrong with the calculation! Smf115 (talk) 21:58, 16 May 2019 (BST)
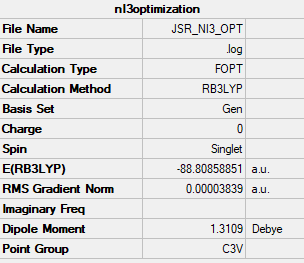
NI3
Item Value Threshold Converged? Maximum Force 0.000063 0.000450 YES RMS Force 0.000038 0.000300 YES Maximum Displacement 0.000480 0.001800 YES RMS Displacement 0.000279 0.001200 YES
Low frequencies --- -12.7477 -12.7416 -6.4258 -0.0140 0.0210 0.0991 Low frequencies --- 101.0290 101.0295 147.4197
optimised NI3 molecule |
The optimised distance for the N-I bond was calculated to be 2.184 Å
Ionic Liquids
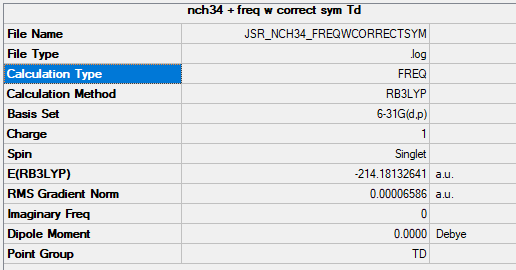
[N(CH3)]4+
Item Value Threshold Converged? Maximum Force 0.000068 0.000450 YES RMS Force 0.000027 0.000300 YES Maximum Displacement 0.000155 0.001800 YES RMS Displacement 0.000067 0.001200 YES
Low frequencies --- 0.0005 0.0007 0.0008 35.2305 35.2305 35.2305 Low frequencies --- 218.8080 317.4871 317.4871
link: https://wiki.ch.ic.ac.uk/wiki/images/0/09/JSR_NCH34_FREQWCORRECTSYM.LOG
optimised [N(CH3)4]+ molecule |
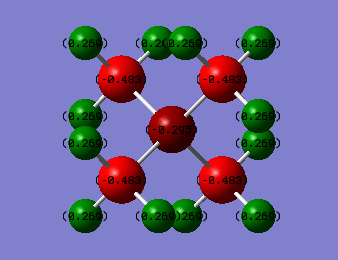
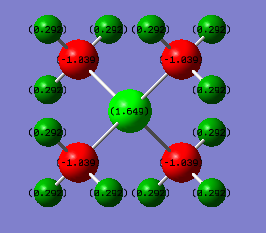
The positive formal charge on the Nitrogen atom in [N(CH3)]4+ comes from the fact that the nitrogen uses its lone pair of electrons to make a dative bond to the electron deficient boron centre losing its electron density thus creating a positive charge on the atom. This is also shown by 4 electron domains being around the nitrogen, one electron domain more it would accommodate if it were neutral. However the positive charge present would more likely be located and spread out over the most electropositive atoms in the molecule system which are the hydrogens on the methyl groups.
You've tried to explain the traditional picture but considering formal electron counting would have helped. Considering N(Me)3, then there are also 4 'electron domains' around the N here but no formal charge on the N, this is because one is a lone pair, it is further detail like this which you should consider further. Smf115 (talk) 21:33, 19 May 2019 (BST)
Charge Distribution Diagram for [N(CH3)]4+:
3 Valence Molecular Orbitals for [N(CH3)]4+
You've chosen a good range of MOs which would have been improved if you'd tried to identify the interactions or overall character of them. A good attempt at constructing the FOs and corresponding LCAOs however, they are incorrect for MO 10 and while 18 has the right AOs, it has been poorly drawn so is hard to identify. Considering the BH3 MO diagram might help in constructing and drawing them. Smf115 (talk) 20:57, 21 May 2019 (BST)
[P(CH3)]4+
Item Value Threshold Converged? Maximum Force 0.000030 0.000450 YES RMS Force 0.000012 0.000300 YES Maximum Displacement 0.000107 0.001800 YES RMS Displacement 0.000044 0.001200 YES
Low frequencies --- 0.0000 0.0017 0.0021 50.8172 50.8172 50.8172 Low frequencies --- 186.8946 211.7454 211.7454
optimised [P(CH3)4]+ molecule |
Charge Distribution Diagram for [P(CH3)]4+:
You've correctly calculated the structure and the NBO charges which is good. The order of the second section hasn't been thought out and you've made no attempt to discuss the charge distributions. The frequency log file for [P(CH3)]4+ is also missing here. Smf115 (talk) 21:36, 19 May 2019 (BST)
Overall, an ok report with good consideration given to accuracy throughout. Your charge analysis part of the project section sadly let you down quite a bit. Smf115 (talk) 20:57, 21 May 2019 (BST)