Rep:Mod:Joelswiki
NH3 molecule
NH3 bond distance = 1.01798
NH3 bond angle = 105.749°
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy = -56.55776873 a.u.
Point Group: C3V
| Item | Value | Threshold | Converged? | |
|---|---|---|---|---|
| Maximum | Force | 0.000004 | 0.000450 | YES |
| RMS | Force | 0.000004 | 0.000300 | YES |
| Maximum | Displacement | 0.000072 | 0.001800 | YES |
| RMS | Displacement | 0.000035 | 0.001200 | YES |
NH3 molecule |
The optimisation file is linked to here
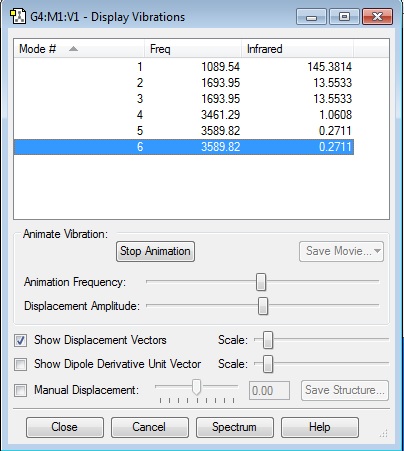
1. How many modes do you expect from the 3N-6 rule? Since N = 4, 3(4) - 6 = 6 modes expected
2. Which modes are degenerate (ie have the same energy)? Modes 2/3 and 5/6 are degenerate
3. Which modes are "bending" vibrations and which are "bond stretch" vibrations? Modes 1-3 are bends while 4-6 are stretches
4. Which mode is highly symmetric? Mode 4 (Symmetric bond stretch)
5. One mode is known as the "umbrella" mode, which one is this? Mode 1
6. How many bands would you expect to see in an experimental spectrum of gaseous ammonia? 2 bands. Mode 4,5,6 are weak signals and will not be clearly peaked in the spectrum. Of the remaining vibrational modes, one band will be from mode 1, while the other will be from the degenerate modes 2 and 3
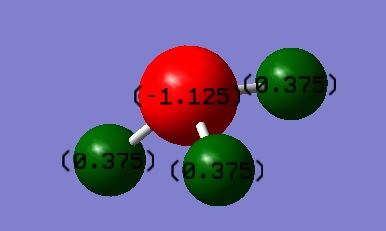
N: -1.125
H: 0.375
N is expected to have the negative charge as it is more electronegative than H, which is thus expected to have a positive charge
H2 molecule
H2 bond distance = 0.74289
H2 bond angle = 180°
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy = -1.17853935 a.u.
Point Group: D∞h
| Item | Value | Threshold | Converged? | |
|---|---|---|---|---|
| Maximum | Force | 0.000066 | 0.000450 | YES |
| RMS | Force | 0.000066 | 0.000300 | YES |
| Maximum | Displacement | 0.000087 | 0.001800 | YES |
| RMS | Displacement | 0.000123 | 0.001200 | YES |
H2 molecule |
The optimisation file is linked to here
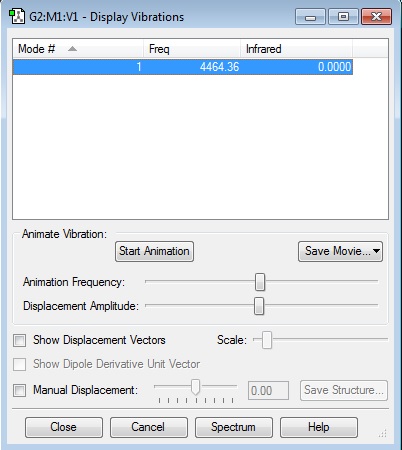
Vibrational mode: Bond stretch (4464.36 cm-1). No bands will form on an IR spectrum (Infrared value of 0.000) as H2 is a linear homodinuclear molecule with no net dippole moment.
N2 molecule
N2 bond distance = 1.10550
N2 bond angle = 180°
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy = -109.52412868 a.u.
Point Group: D∞h
| Item | Value | Threshold | Converged? | |
|---|---|---|---|---|
| Maximum | Force | 0.000001 | 0.000450 | YES |
| RMS | Force | 0.000001 | 0.000300 | YES |
| Maximum | Displacement | 0.000000 | 0.001800 | YES |
| RMS | Displacement | 0.000000 | 0.001200 | YES |
N2 molecule |
The optimisation file is linked to here
Vibrational mode: Bond stretch (2457.33cm-1). No bands will form on an IR spectrum (Infrared value of 0.000) as N2 is a linear homodinuclear molecule with no net dippole moment.
Reaction energy of the Haber-Bosch process
N2 + 3 H2 --> 2 NH3
E(NH3)= -56.55776873 a.u.
2*E(NH3)= -113.115537 a.u.
E(N2)= -109.52412868 a.u.
E(H2)= -1.17853935 a.u.
3*E(H2)= -3.53561805 a.u.
ΔE= Energy of products - Energy of reactants =2*E(NH3)-[E(N2)+3*E(H2)]= -0.05579095 a.u. = -146.48 kJ/mol (Literature value: -92.4 kJ/mol)
As the reaction is exothermic, the product(ammonia) is more stable than the reactants(nitrogen and hydrogen).
Choice of molecule: O2
O2 bond distance = 1.21461
Calculation Method: RB3LYP
Basis Set 6-31G(d,p)
Final Energy = -150.32004019 a.u.
Point Group: D∞h
| Item | Value | Threshold | Converged? | |
|---|---|---|---|---|
| Maximum | Force | 0.000114 | 0.000450 | YES |
| RMS | Force | 0.000114 | 0.000300 | YES |
| Maximum | Displacement | 0.000069 | 0.001800 | YES |
| RMS | Displacement | 0.000097 | 0.001200 | YES |
O2 molecule |
The optimisation file is linked to here
Vibrational mode: Bond stretch (1658.53 cm-1). No bands will form on an IR spectrum (Infrared value of 0.000) as O2 is a linear homodinuclear molecule with no net dippole moment.
Both O: 0.000
Net charge of 0 on both oxygen atoms is expected as O2 is a linear, homodinuclear molecule and both atoms have the same electronegativity.
Molecular Orbitals
Spin: Triplet





Extra Information
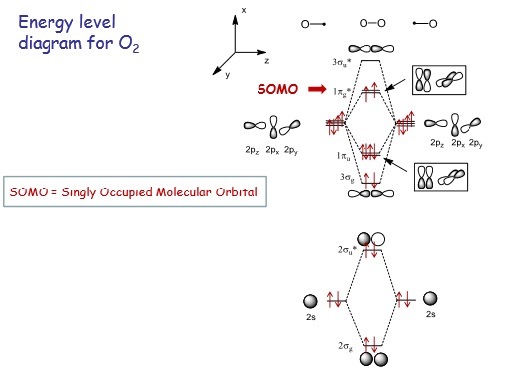
Credit: Prof. Ramon Vilar, Imperial College London
From the experimental MO diagram of O2 the π bonding orbitals (1πu) are higher in energy than the σ bonding orbital (3σg) as molecular orbital mixing is not significant enough to raise the energy of the 3σg orbital above the 1πu orbital (as the 2σg and 3σg MOs are not close enough in energy). This is in contrast to the theoretically calculated MO from gaussian which puts the 3σg orbital at a higher energy level