Rep:Mod:JesseFelix1
The Hydrogenation of a Cyclopentadiene Dimer
A molecular mechanics (MM) model energy minimisation was carried out for the exo and endo isomers of a cyclopentadiene dimer. The same was done for the two possible products of one of the two C=C bonds being hydrogenated.
| Isomer | Energy(kcal/mol) | |||||||
| Str | Bnd | Str-Bnd | Tor | Non-1,4 VdW | 1,4 VdW | H-Bond | Total | |
| Exo isomer | 1.2836 | 20.5796 | -0.8376 | 7.6564 | -1.4172 | 4.2342 | 0.3775 | 31.8765 |
| Endo isomer | 1.2505 | 20.8472 | -0.8354 | 9.5113 | -1.5449 | 4.3211 | 0.4477 | 33.9975 |
| Dihydro product 1 | 1.2781 | 19.8597 | -0.8346 | 10.8110 | -1.2243 | 5.6329 | 0.1621 | 35.6850 |
| Dihydro product 2 | 1.0967 | 14.5258 | -0.5494 | 12.4978 | -1.0714 | 4.5118 | 0.1406 | 31.1520 |
This shows that the exo dimer is the thermodynamically favourable product, while the endo is the kinetic product. This can be explained by looking at the new bonds. In the exo version the two larger groups are antiperiplanar, while in the endo version they are synperiplanar. For this reason the reaction must be a kinetically-controlled cycloaddition (the endo is the more abundant product).
Dihydro product 2 is thermodynamically more favourable than dihydro product 1. The most significant energy differences involved are the bend, torsion and 1,4 VdW energies which are -5.34, +1.69 and -1.12kcal/mol respectively (from product 1 to product 2). The one increased energy (Torsion energy) is likely explained by the ring strain on a cyclopentene system vs a cyclopentane system. All the other energy differences are negligible.
Atropisomerism in a synthetic intermediate of Taxol
Two atropisomers of a synthetic intermediate of Taxol exist - depending on whether the carbonyl group points up (1) or down (2) relative to the ring system - and have had their energies minimised using the MM2 and MMF94 force-fields:
| Atropisomer | Energy (kcal/mol) | ||||||||
| Str | Ben | Str-Ben | Tor | Non-1,4 VdW | 1,4 VdW | H-bond | MM2 Total | MMF94 Total | |
| Atrop. 1 (MM2/MMF94) | 2.7828 | 16.5415 | 0.4300 | 18.2555 | -1.5567 | 13.1115 | -1.7251 | 47.8396 | 70.5349 |
| Atrop. 2 (MM2/MMF94) | 2.5672 | 13.4359 | 0.3122 | 17.6727 | -1.5469 | 12.4636 | -1.5943 | 43.3104 | 61.0722 |
Both atropisomers can be classed as 'hyperstable alkenes'[1] because of the cage-like formation protecting their respective C=C bonds and their reduced calculated energy versus that of their otherwise analogous alkane form. The hydrogenated version of atropisomer 1 has a calculated total energy of 62.5005 kcal/mol and that of atropisomer 2 is 63.0890 kcal/mol. This increase in energy can be explained by the increased strain in the ring system caused by changing the two C's in the C=C bond from sp2 to sp3 bond geometry.
The MMF94 force-field calculates higher total energies than MM2 because it does not take into account the 'olefinic strain' that is also responsible for making the alkene system hyperstable.
Regioselective Addition of Dichlorocarbene to Diene


Diene A was minimised for energy using the MM2 force-field and the MOPAC electronic structure method and the two were compared by superimposing the molecules on one another. The disparity between the two molecules is the greater nearer the chlorine molecule, which is an obvious conclusion as the MOPAC method takes into account electronic effects. More interestingly though, the disparities observed between the molecules are greater on the cyclohexene on the chlorine's 'side' of the molecule than on the hydrogen's.
| Table 3.1 - Molecular Disparity of A | |
| Molecule Pair | Measured Distance (Å) |
| C(1)-C(1)' | 0.0229 |
| C(2)-C(2)' | 0.1413 |
| C(3)-C(3)' | 0.0976 |
| C(4)-C(4)' | 0.1187 |
| C(5)-C(5)' | 0.1226 |
| Cl-Cl' | 0.1351 |
The greatest C(n)-C(n)' difference is between the 2-position carbons, which are on the hydrogen 'side' of the molecule - larger than the difference between the 4-position carbons on the otherwise analogous carbons on the chlorine side. However the difference between the 5-carbons is significantly greater than that between the 1's. This could be explained by the greater size of chlorine, i.e. its effect reaches further, versus the increased charge density of hydrogen, causing it to have a greater effect on atoms that are close enough. Furthermore, the chlorine is highly electronegative meaning its effect on the position of the carbons involved in the C=C bond would be greater because of the relatively delocalised electrons forming the π-bond. The Cl-Cl' difference is one of the greatest in the molecule showing that electronic effects are playing a significant part in the MOPAC's altered arrangement of A.
Monosaccharide Chemistry and Glycosidation
Isomer 1 (MM2/MOPAC(PM6))
------------MM2 Minimization------------ Warning: Some parameters are guessed (Quality = 1). Iteration 753: Minimization terminated normally because the gradient norm is less than the minimum gradient norm Stretch: 2.4883 Bend: 10.0123 Stretch-Bend: 0.8505 Torsion: 4.8596 Non-1,4 VDW: -2.3299 1,4 VDW: 20.4846 Charge/Dipole: -11.2819 Dipole/Dipole: 4.8597 Total Energy: 29.9431 kcal/mol Calculation completed ------------------------------------ ------------ Mopac Interface ------------ Model: Jesse_monosacharride_LeftDown.mol Mopac Job: AUX PM6 CHARGE=1 EF GNORM=0.100 SHIFT=80 Finished @ RMS Gradient = 0.09284 (< 0.10000) Heat of Formation = -85.04730 Kcal/Mol -----------------------------------------
Mini Project
Practice: Taxol Intermediate
 |
A molecule A was modelled and optimised using the MM2 method:
------------MM2 Minimization------------ Warning: Some parameters are guessed (Quality = 1). Iteration 2: Minimization terminated normally because the gradient norm is less than the minimum gradient norm Stretch: 5.3666 Bend: 20.2633 Stretch-Bend: 0.8831 Torsion: 22.5577 Non-1,4 VDW: -0.3472 1,4 VDW: 17.4114 Dipole/Dipole: -1.7705 Total Energy: 64.3644 kcal/mol Calculation completed ------------------------------------
It was then further optimised using a Gaussian method with the basis set 6-31G(d,p). The now fully optimised molecule was then re-submitted for an NMR calculation(DOI:10042/23269 ). The resulting spectra are as follows:
 |
 |
Compared to the literature [2], the 1H spectrum is not a very good match. The 13C spectrum, however, is actually very comparable to the values recorded in the existing paper.
Reactive preference of (Z)-1,1-Diphenylbut-2-ene over its E- isomer
Introduction
The varied substitutions of α-substituted allylboronates is currently a field of some interest[3] particularly those with the pinacolatoboron moiety.
The reaction is appropriate for the mini-project as the original paper has recorded 1H NMR, 13C NMR and mass spectra available in its supporting information section. Furthermore, there is very little (in fact almost no) conformational flexibility as the bulk of the molecule is the 2 benzene rings. The remaining alkyl section is broken up by the defining C=C bond. It is also small and simple enough for the MM2 method to provide a good geometrical optimisation.
Results and Discussion
 |
 |
Molecules of B (6.8319 kcal/mol) and C (4.5066 kcal/mol) were first optimised using the MM2 method.
From this reasonably good approximatons were found for the molecules due to the compound's relatively small size and its simplicity. However, a subsequent geometric optimisation was carried out on the SCAN system using the DFT method MPW1PW91 with a 6-31G(d,p) basis set. Further refined models for B and C were obtained.
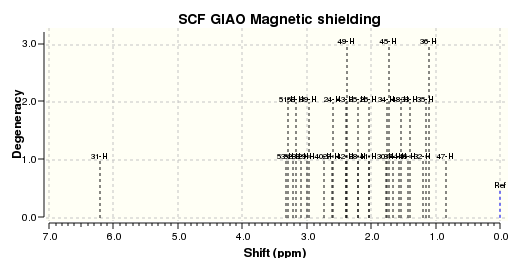
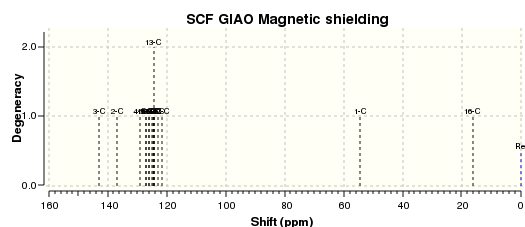
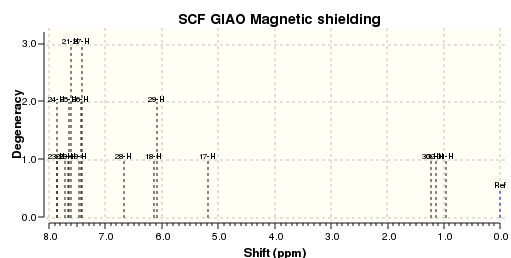
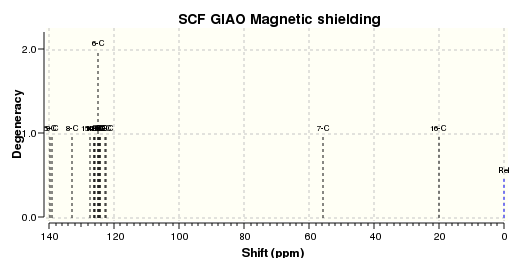
An NMR calculation was then carried out for the Z (DOI:10042/23259 ) and E (DOI:10042/23261 ) isomers and spectra for their respective 13C and 1H were obtained:
 |
 |
 |
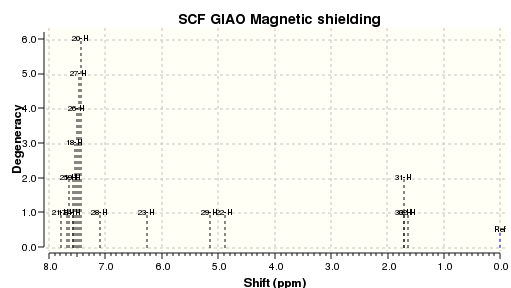 |
The literature 13C spectrum is a reasonably good match to the two calculated ones. However many of the signals are over 5ppm out. This could be because the spectrum obtained in the original research was some mixture of the two isomers. The 1H spectrum in the paper is not a particularly good match to either of the two computed.
Frequency analysis
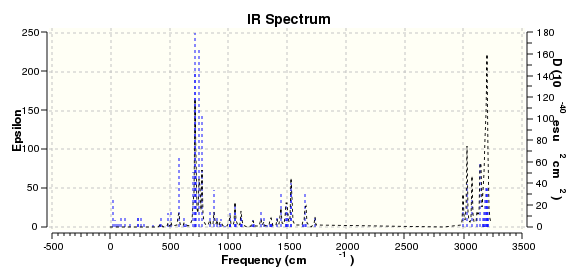
A frequency analysis was then carried out for B (DOI:10042/23275 ) and C(DOI:10042/23276 ) and simulated IR spectra were obtained:
 |
 |
None of the modes of frequency came back as negative so the resulting molecule is not a transition state. Unfortunately there are no literature IR spectra to compare these to. However, the two isomers' ΔG values can be compared to one another:
| Isomer | ΔG(Hartrees) |
| B | -619.112261 |
| C | -619.119114 |
This shows that the E-isomer has a (very slightly) more negative Gibbs Free Energy than the Z-isomer. This tallies with the previous MM2 energy minimisation - which showed that the E-isomer had a lower overall energy - to demonstrate that the E-isomer is the thermodynamically favourable product. However, this isomer only makes up 3% of the product yield. From this, the conclusion can be drawn that the reaction itself is kinetically controlled.
Further calculations
Various other calculations were carried out on the isomers. However, with no corresponding literature to compare to, the simple D-space publications are presented here:
| Supporting calculations | ||
| B | C | |
| Circular Dichroism | DOI:10042/23283 | DOI:10042/23284 |
| Molecular Orbitals | DOI:10042/23285 | DOI:10042/23286 |
References
- ↑ W.F. Maier, P.R. Schleyer, J. Am. Chem. Soc., 1981, 103, 1891-1900: DOI:10.1021/ja00398a003
- ↑ Paquette, N. A. Pegg, D. Toops, G. D. Maynard, R. D. Rogers, J. Am. Chem. Soc.,, 1990, 112, 277-283. DOI:10.1021/ja00157a043
- ↑ A. Mace, F. Tripoteau, Q. Zhao, E. Vrancken, J.-M. Campagne, B. Carboni, Organic Letters,2013,: DOI:10.1021/ol4000263
