Rep:Mod:JakesReport
NH3 Molecule
N-H bond distance= 1.01798 Angstrom H-N-H bond angle= 105.741 degrees
The literature value for the N-H bond distance is 1.012 Angstrom and the bond angle is 106.7 degrees. [1]
Molecule: NH3 Calculation Method: RB3LYP Basis Set: 6-31G(d,p) Final Energy E(RB3LYP): -56.55776873 a.u. RMS Gradient: 0.00000485 Point Group: C3V
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
NH3 molecule |
The optimisation file is liked to here
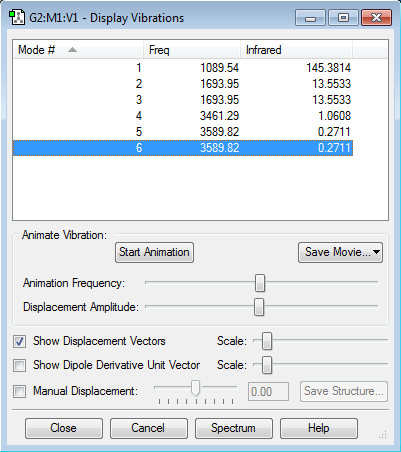
There are no negative frequencies.
how many modes do you expect from the 3N-6 rule? 6 which modes are degenerate (ie have the same energy)? 2 and 3, 5 and 6 which modes are "bending" vibrations and which are "bond stretch" vibrations? Bending:1,2,3 Stretching:4,5,6 which mode is highly symmetric? 4 one mode is known as the "umbrella" mode, which one is this? 4 how many bands would you expect to see in an experimental spectrum of gaseous ammonia? 4
Charge on the Nitrogen= -1.125 Charge on the Hydrogens= 0.375
This is expected as nitrogen is very electronegative and so would have a negative value and hydrogen would be positive.
N2 Molecule
Molecule: N2 Calculation Method: RB3LYP Basis Set: 6-31G(d,p) Final Energy E(RB3LYP): -109.52359111 a.u. RMS Gradient: 0.02473091 Point Group: D∞h
N-N bond distance= 1.09200 Angstrom
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
N2 molecule |
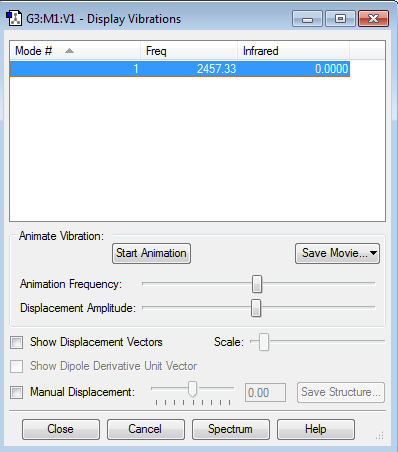
There are no negative frequencies.
The optimisation file is liked to here
how many modes do you expect from the 3N-5 rule? 1 which modes are "bending" vibrations and which are "bond stretch" vibrations? 1 is a stretch which mode is highly symmetric? 1
The charge on each nitrogen was 0.000
H2 Molecule
Molecule: H2 Calculation Method: RB3LYP Basis Set: 6-31G(d,p) Final Energy E(RB3LYP): -1.17853936 a.u. RMS Gradient: 0.00000017 Point Group: D∞h
H-H bond distance= 0.74279 Angstrom
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
H2 molecule |
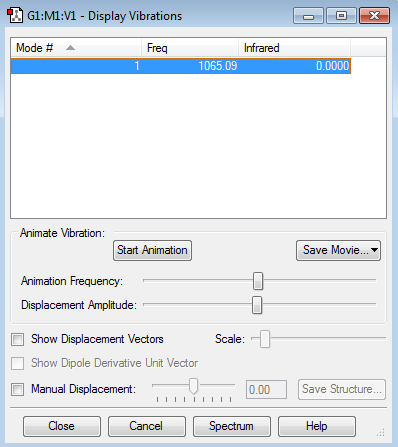
There are no negative frequencies.
The optimisation file is liked to here
how many modes do you expect from the 3N-5 rule? 1 which modes are "bending" vibrations and which are "bond stretch" vibrations? 1 is a stretch which mode is highly symmetric? 1
The charge on each hydrogen was 0.000
Reactivity
E(NH3)=-56.55776873 au 2*E(NH3)=-113.11553746 au E(N2)= -109.52359111 au E(H2)= -1.17853936 au 3*E(H2)=-3.53561808 au ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.05616555 au ΔE=-147.46 kJ/mol
The products are more stable than the reactants.
F2 Molecule
Molecule: F2 Calculation Method: RB3LYP Basis Set: 6-31G(d,p) Final Energy E(RB3LYP): -199.49825218 a.u. RMS Gradient: 0.00007365 Point Group: D∞h
F-F bond distance=1.40281 angstrom
Item Value Threshold Converged? Item Value Threshold Converged? Maximum Force 0.000128 0.000450 YES RMS Force 0.000128 0.000300 YES Maximum Displacement 0.000156 0.001800 YES RMS Displacement 0.000221 0.001200 YES
F2 molecule |
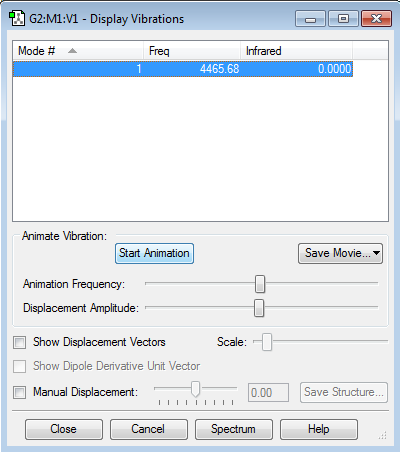
There are no negative frequencies.
The optimisation file is liked to here
The charge on each flourine was 0.000
how many modes do you expect from the 3N-5 rule? 1 which modes are "bending" vibrations and which are "bond stretch" vibrations? 1 is a stretch which mode is highly symmetric? 1
F2 Molecular Orbitals
MO1 is a bonding orbital with electrons from the 2s orbital on each F, however due to a σ* from the 2s orbital in each F, it does not contribute to bonding overall. MO2 is a σ* orbital from the 2s orbital in each F, it is higher in energy than the MO1 but lower than MO3, MO4 and MO5. MO1 is the deepest in energy out of the 5 MO's shown above. MO3 is a σ orbital with electrons from a 2p orbital on each fluorine, it contributes to bonding as there is not a σ* from the 2p orbital negate it. MO4 and MO5 are both π orbitals with electrons from a 2p orbital on each fluorine. They do not contribute to bonding due to there being 2 π* MO's.