Rep:Mod:JZ01554120
1. Ammonia molecules analysis
| molecule name | NH3 |
| calculation method | RB3LYP |
| basis set | 6-31G(d,p) |
| final energy | -56.55776873 a.u. |
| point group | C3V |
| bond length | 1.02±0.01Å |
| bond angle | 106±1° |
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000070 0.001800 YES RMS Displacement 0.000033 0.001200 YES
ammonia molecule |
The optimisation file is liked to here
spectroscopic properties

| Models | 1 | 2 | 3 | 4 | 5 | 6 |
| wavenumber/cm-1 | 1090 | 1694 | 1694 | 3461 | 3590 | 3590 |
| Symmetry | A1 | E | E | A1 | E | E |
| intensity/arbitrary units | 145 | 14 | 14 | 1 | 0 | 0 |
| image | 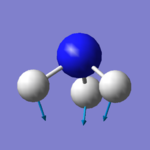 |
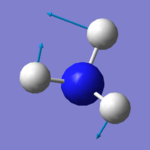 |
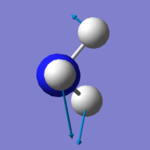 |
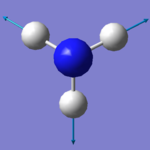 |
 |
|
There are six expected modes of ammonia molecule according to the 3N-6 rule and four modes are degenerated ( two-two each). According to the table above, mode 3 and 4 are a pair of degenerated modes and mode 5 and 6 are another pair as they have same wavenumbers. The first three modes are all bending vibrations and last three are stretching vibrations. Mode 1 and 4 are highly symmetry, as they include point group A1, the other four modes only have E (identity). Mode 4 is called the umbrella mode. four brands are expected to see in a spectrum.
Atomic charge of ammonia molecule
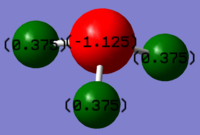
The charge of nitrogen atom is -1.125 and for each hydrogen atom is 0.375. The charges are expected to be 0.230 (positive) for hydrogen and -0.690 (negative) for nitrogen from calculation through dipole moment, and because nitrogen is more electronegative compare with hydrogen, it is expected to carry the negative charge.
Hydrogen molecule analysis
| molecule name | H2 |
| calculation method | RB3LYP |
| basis set | 6-31G(d,p) |
| final energy | -1.15928020 a.u. |
| point group | D∞h |
| bond length | 0.60±0.01Å |
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
hydrogen molecule |
The optimisation file is liked to here
spectroscopic properties
| Models | 1 |
| wavenumber/cm-1 | 4466 |
| Symmetry | SGG |
| intensity/arbitrary units | 0 |
| image | 
|
3N-5 rule is applied here as hydrogen is a linear molecule, and only one vibration mode of stretching vibration is expected to see.
Charge of H2 molecule

Atomic charge of hydrogen is 0 as it is a uniatomic molecule and the two H atoms have same electronegativity.
Nitrogen molecule analysis
| molecule name | N2 |
| calculation method | RB3LYP |
| basis set | 6-31G(d,p) |
| final energy | -109.52359111 a.u. |
| point group | D∞h |
| bond length | 1.09±0.01Å |
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
nitrogen molecule |
The optimisation file is liked to here
Spectroscopic analysis of nitrogen
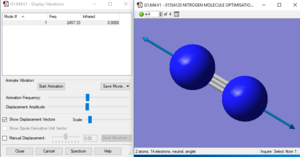
| Models | 1 |
| wavenumber/cm-1 | 2457 |
| Symmetry | SGG |
| intensity/arbitrary units | 0 |
| image | 
|
There will be only one absorption according to the 3N-5 rule for linear molecule.
Charge analysis of nitrogen
The two nitrogen atoms have same electronegativity, so the charge are evenly separated and both are 0 on each atom.
Transition metal complex
| Name | (dinitrogen)-(2,6-bis(1-(2,6-diisopropylphenylimino)propyl)pyridine)-iron |
| Code | BARTOF |
| N-N bond length | 1.112Å |
| image | 
|
The structure can be found [here]
Bond length between the two nitrogen atom is longer compare with the N-N bond in nitrogen molecule. This is because when the nitrogen molecule bonded with a electron rich transition element like Fe, electron density is pushed into the anti-bonding π* orbital and weaken the N-N bond. therefore, the bond length of N-N bond will increase when it for complex ions. it also because the electron density becomes diffused when a new bond is formed between nitrogen molecule and a metal atom. In this situation, as the value is obtained from computational calculation, there may also be certain degree of inaccuracy.
reaction energy of Haber-Bosch Process
E(NH3)=-56.5577687 2*E(NH3)=-113.1155374 E(N2)=-109.5235911 E(H2)=-1.1592802 3*E(H2)=-3.4778406 ΔE=2*E(NH3)-[E(N2)+3*E(H2)]=-113.1155374-[-109.5235911-3.4778406]=-0.1141057 ΔE=2625.5*-0.1141057=-299.6kjmol-1
The reaction energy has a negative value, which suggests the product (ammonia) is more stable compare with the reactant (hydrogen and nitrogen).
Analysis of ammonium ion
| molecule name | NH4+ |
| calculation method | RB3LYP |
| basis set | 6-31G(d,p) |
| final energy | -56.89738175 a.u. |
| point group | C1 |
| bond length | 1.03±0.01Å |
| bond angle | 109±1° |
Item Value Threshold Converged? Maximum Force 0.000203 0.000450 YES RMS Force 0.000097 0.000300 YES Maximum Displacement 0.001016 0.001800 YES RMS Displacement 0.000578 0.001200 YES
ammonium ion |
The optimisation file is liked to here
spectroscopy analysis of ammonium ion
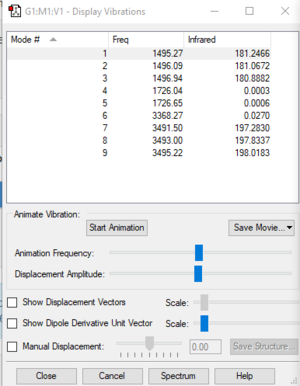
| Models | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| wavenumber/cm-1 | 1495 | 1496 | 1497 | 1726 | 1727 | 3368 | 3491 | 3493 | 3495 |
| Symmetry | A | A | A | A | A | A | A | A | A |
| intensity/arbitrary units | 181 | 181 | 181 | 0 | 0 | 0 | 197 | 198 | 198 |
| image |  |
 |
 |
 |
 |
 |
 |
 |
|
According to the 3N-6 rule, there are 9 vibration modes according to the 3N-6 rule for non-linear molecules. Therer will be four distinguishable peaks on the spectrum as some of those modes are degenerated.
Charge analysis of ammonium ion
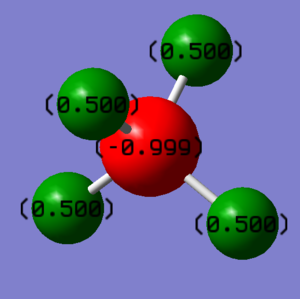
Nitrogen atom bare negative charge and hydrogen atoms bear positive charge, this is because the electronegativity of nitrogen is bigger than hydrogen. The overall charge is not 0 as the ion is not a neutral species and it has +1 overall charge.
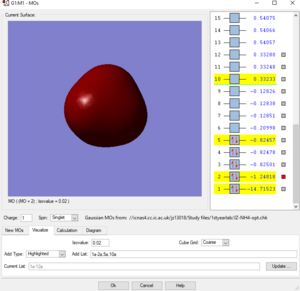
Molecular orbital analysis of ammonium ion
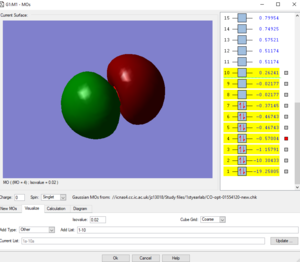
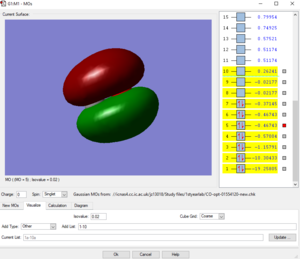
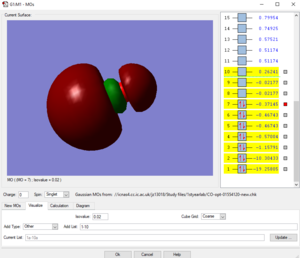
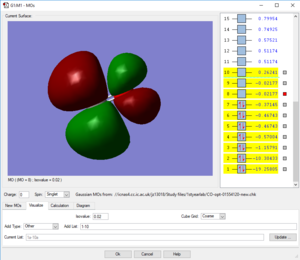
Picture1: This is the lowest energy orbital of NH4+ ion, it is surrounded to the nitrogen atom, means it is the 1s orbital of nitrogen atom, its energy is very low (-14.71523 a.u.) so it cannot be included in bounding. This orbital doesn't contribute to the bonding.
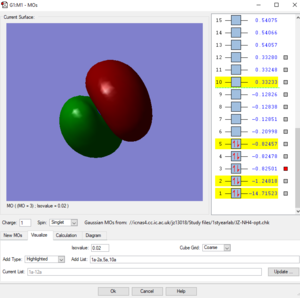
Picture2: This is the second lowest energy orbital (-1.24818 a.u.). The AOs contributed to it are the 2s orbital of nitrogen and the mixed orbital from the 4 1s orbitals of the four hydrogen atoms.This orbital is the bonding orbital of the overlapping. This orbital contributes to the bonding.
Picture3: This one of the bonding orbitals of ammonium ion, it is form by a nitrogen 2p orbital over with a hydrogen AO. This orbital contributes to the bonding.
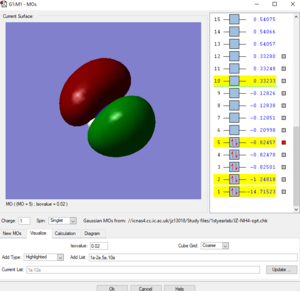
Picture4: This is the HOMO of the ammonium ion, it is the anti-bonding orbital of the overlap between 2s orbital of nitrogen and one of the four hydrogen atomic orbitals. Due to stablisation, the HOMO is quite close in energy (-0.82457 a.u.) with the previous 2 orbitals, not like the HOMO of ammonium molecule. This orbital contributes to the bonding.
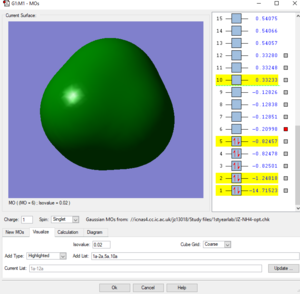
Picture5: This is the LUMO of the ammonium ion, its energy (-0.20998 a.u.)is higher than the previous three molecular orbitals (approx-0.825 a.u.). and it is made from a p orbital of nitrogen and one of the four 4H atomic orbitals. This orbital doesn't contribute to the bonding.
Analysis of CO molecule
| molecule name | CO |
| calculation method | RB3LYP |
| basis set | 6-31G(d,p) |
| final energy | -113.30945314 a.u. |
| point group | C∞v |
| bond length | 1.14±0.01Å |
Item Value Threshold Converged? Maximum Force 0.000032 0.000450 YES RMS Force 0.000032 0.000300 YES Maximum Displacement 0.000012 0.001800 YES RMS Displacement 0.000018 0.001200 YES
carbon monoxide molecule |
The optimisation file is liked to here
Spectroscopy analysis of carbon monoxide
| Models | 1 |
| wavenumber/cm-1 | 2209 |
| Symmetry | SG |
| intensity/arbitrary units | 67 |
| image | 150px |
Charge analysis of carbon monoxide
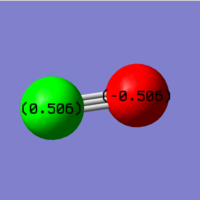
As carbon and oxygen atoms have different electronegativity, charge will distributed unevenly on two atoms, and as oxygen is more electronegative, it will carry the negative charge.
molecular orbital of carbon monoxide

Picture1: This is the bonding orbital (3σ) formed by the overlap of two 2s orbital of carbon and oxygen atom, its energy is -1.15791 a.u. This orbital contributes to the bonding.
Picture2: This is the 4σ orbital of the molecule, it is made by two head to head 2p orbitals overlap in phase. Its energy is -0.57004a.u. This orbital contributes to the bonding.
Picture3: This is one of the degenerated 1π orbitals of the molecule, it is formed by two 2p orbital of each atom in phase parallel overlap and its energy is -0.46743 a.u. This orbital contributes to the bonding.
Picture4: This is the 5σ orbital of the molecule, it is also the HOMO (energy=-0.37145). it is made by two out of phase 2s orbital from each atom overlap. This orbital doesn't contribute to the bonding.
Picture5: This is one of the degenerated 2π orbital of the molecule, it is also the LUMO (energy=-0.2177 a.u.). it is made by two out of phase 2p from each atom orbital parallel overlap. This orbital doesn't contribute to the bonding, as no electrons in it.
Marking
Note: All grades and comments are provisional and subjecct to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have recieved your grade from blackboard.
Wiki structure and presentation 1/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES -however, you could have used subsections more efficiently
Do you effectively use tables, figures and subheadings to communicate your work?
YES
NH3 0.5/1
Have you completed the calculation and given a link to the file?
YES
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES - You identified the requirement for degenerate vibrations correctly. However, you made a mistake regarding the degenerate modes. Modes 2 and 3 are degenerate, not 3 and 4. You stated mode 4 to be the umbrella mode but from optical inspection of this mode t is obvious this is a symmetric stretch of all three NH bonds. Mode 1 is the umbrella mode as it resembles the motion when opening an umbrella. You correctly stated that there are two sets of degenerate modes - this explains a spectrum with 4 peaks. However there are only 2 peaks visible as peaks 4, 5 and 6 are of too low an intensity to be visible.
N2 and H2 0.5/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
YES - however you stated H2 to be a uniatomic molecule, this would be one atom! H2 is a diatomic with both atoms being the same.
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES
Have you compared your optimised bond distance to the crystal structure bond distance?
YES
Haber-Bosch reaction energy calculation 0.5/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
NO - The reported number of H2 is incorrect by 0.2 a.u., although the linked .log file contains the right energy for optimised H2. This results in a wrong overall reaction energy.
Have you reported your answers to the correct number of decimal places?
YES
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES
Your choice of small molecule 2.5/5
Have you completed the calculation and included all relevant information?
YES - however the point group of NH4+ is expected to be Td rather than C1.
You tried to comment on the vibrations. You missed to state how many sets of degenerate modes were calculated and which modes are included to these sets. The modes 1-3, 4 &5 and 7-9 are degenerate . This would explain a spectrum with 4 peaks. However the intensities of the modes 4, 5 and 6 are too low in intensity to be observed experimentally. Therefore only two peaks will be seen in an experimental spectrum.
Have you added information about MOs and charges on atoms?
You have done a good job of presenting this information, well done!
In most cases you stated the contributing AOs correctly and only missed to mention them explicitly once. You missed to state which orbital is occupied/non-occupied. The MOs 3-5 should all be degenerate and all bonding MOs. This discrepancy probably derives from the C1 point group of your ammonium ion. The LUMO has no contribution from a p orbital. It is an ou-of-phase combination of the 2s on N and the 1s on the Hs and resembles a non-bonding MO with an internal node.
Independence 1/1
If you have finished everything else and have spare time in the lab you could: Check one of your results against the literature, or Do an extra calculation on another small molecule, or
YES - well done!
Do some deeper analysis on your results so far
