Rep:Mod:JPW1912
Ammonia and the Haber-Bosch Reaction
NH3
The molecule is Ammonia, formula NH3. The calculation method was RB3LYP with a basis set of 6-31G(d.p).
Key Data
Final Energy - -56.55664124 au
RMS Gradient - 0.00836082 au
Point Group - C3V
N-H bond length - 1.00000 angstroms
H-N-H bond angle - 109.471 degrees
Item Table
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000014 0.001800 YES RMS Displacement 0.000009 0.001200 YES
Jmol
NH3 |
Vibrations
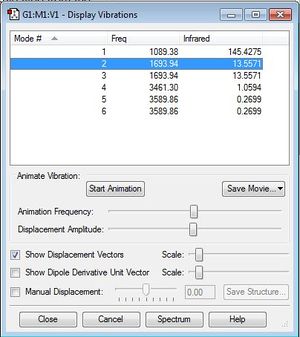
NH3 is non-linear and therefore it follows the 3N-6 rule. As there are 4 atoms, it is expected that there are 6 normal modes of vibration. There are 6 modes of vibration in the Display Vibrations window which matches the expected result. Of the observed vibrations, they are split into 4 distinct groups, 2 of which contain 2 separate degenerate vibrations. The 2 different asymmetric stretches (5 and 6) are degenerate both with a frequency of 3589.86/s. The other 2 degenerate vibrations are the asymmetric bends (2 and 3) both with a frequency of 1693.94/s. The mode that is very highly symmetric is the symmetric bend through the plane (1) with a frequency of 1089.39/s, this mode is also known as the umbrella mode. An experimental spectrum of gaseous ammonia is expected to have 4 bands.
Charge
The observed charge on the N atom is -1.125 and the charge on each H atom is 0.375. With a total of 3 H and 1 N atoms the total charge on the molecule equals 0. Due to the fact that Nitrogen is much more electronegative than Hydrogen, I would expect that Nitrogen would have a negative charge and that the charge on each Hydrogen would be positive and 1/3 of the absolute value of the charge on the Nitrogen so that the overall charge would be neutral. This expectation is shown in the results.
Optimization CHK File
Link to completed NH3 Optimization - File:JPW115 NH3 OPT.LOG
N2
The molecule is Nitrogen, formula N2. The calculation method was RB3LYP with a basis set of 6-31G(d.p).
Key Data
Final Energy -109.52359111 au
RMS Gradient - 0.02473091 au
Point Group - D*H
N-N bond length - 1.09200 angstroms
Item Table
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
Vibrations
There is one mode of vibration with frequency 2457.33/s, which is positive, however it has an intensity of 0 and therefore is not observed.
Jmol
N2 |
H2
The molecule is Hydrogen, formula H2. The calculation method was RB3LYP with a basis set of 6-31G(d.p).
Key Data
Final energy - -1.15928020 au
RMS Gradient - 0.09719500 au
Point group - D*H
H-H bond length - 0.60000 angstroms
Item Table
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
Vibrations
There is one mode of vibration with frequency 4465.68, which is positive, however it has an intensity of 0 and therefore is not observed.
Jmol
H2 |
The Haber-Bosch Reaction
Energy of Reaction N2 + 3H2 -> 2NH3
E(NH3)= -56.55664124 au
2*E(NH3)= -113.1132825 au
E(N2)= -109.52359111 au
E(H2)= -1.15928020 au
3*E(H2)= -3.4778406 au
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.11185079 au
Therefore in kJ/mol, the energy of reaction = -293.6642491 kJ/mol. This is a negative energy change for the forward reaction, meaning the ammonia product must have a lower energy than the gaseous reactants and therefore the ammonia product must be more stable.
My Molecule - CH4
Optimization
The molecule is Methane, formula CH4. The calculation method was RB3LYP with a basis set of 6-31G(d.p).
Key Data
Final energy was -40.52275298 au
RMS Gradient - 0.00803799 au
Point group - TD
C-H bond length - 1.07000 angstroms
Item Table
Item Value Threshold Converged? Maximum Force 0.000063 0.000450 YES RMS Force 0.000034 0.000300 YES Maximum Displacement 0.000179 0.001800 YES RMS Displacement 0.000095 0.001200 YES
Jmol
CH4 |
Link to Optimization File
Click here
Vibrations

The displayed vibrations of the optimized CH4 molecule show 9 mode of vibration, over 4 distinct frequencies. However 3 of these (4-6), have 0.0000 intensity and are therefore not observed. Modes of vibration 1,2 and 3 are all degenerate with a frequency of 1356.20/s, they are all bending frequencies. The vibrations 7-9 are all degenerate with frequency 3162.33/s and are all bond stretching vibrations.
Charges
Expected
I would expect Carbon to have a small negative charge, which each hydrogen having a very small positive charge of value exactly 1/4 of the absolute value of the charge on the carbon. This is because carbon is more electronegative than hydrogen but still not very electronegative. And as the molecule has no overall charge, the charges of all the individual atoms must therefore add up to 0.
Observed
The observed charge on the carbon is -0.930 and the charge on each hydrogen is +0.233. This matches my observation very closely however the overall charge on the methane is not actually +0.002, meaning according to the results of the approximation, the methane molecule is very slightly positive.
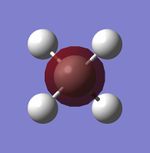
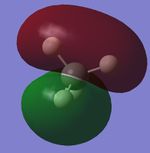
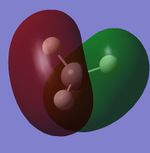
Molecular Orbitals
| CH4 1s Molecular Orbital | CH4 2s Molecular Orbital | CH4 2p Molecular Orbital | CH4 2p Molecular Orbital | CH4 2p Molecular Orbital |
|---|---|---|---|---|
 |
 |
 |
 |
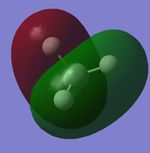
|
Explanations of Molecular Orbitals
The 1s Molecular orbital is a molecular orbital with only carbon contributing and therefore forms the spherical shape of a 1s atomic orbital as shown. The 2s has both hydrogen and carbon contribution, with the carbon 2s and the hydrogen 1s atomic orbitals contributing, however as only s orbitals contribute there are no nodal planes and the MO forms an almost spherical shape. The next 3 Molecular orbitals are all 2p MOs and are contributed to by Carbon 2p Atomic orbitals and Hydrogen 1s atomic orbitals, they have a nodal plane down the center. All orbitals I have shown are bonding molecular orbitals.
