Rep:Mod:JL7816
Inorganic Computation Chemistry
Different molecules
BH3 molecule
B3LYP/6-31G level
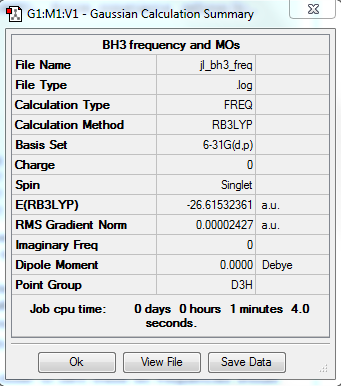
Item Table
Item Value Threshold Converged? Maximum Force 0.000049 0.000450 YES RMS Force 0.000024 0.000300 YES Maximum Displacement 0.000191 0.001800 YES RMS Displacement 0.000095 0.001200 YES
Frequency analysis log file: jl_bh3_freq.log
Low Frequency lines
Low frequencies --- -0.3999 -0.1922 -0.0053 25.7853 27.7381 27.7410 Low frequencies --- 1163.1984 1213.3187 1213.3214
Optimised BH3 molecule |
IR of BH3 molecule
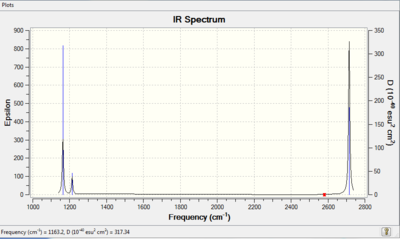
| Mode # | Freq | Intensity | IR active? | Vibrations |
|---|---|---|---|---|
| 1 | 1163 | 93 | YES | angle deformations |
| 2 | 1213 | 14 | YES | angle deformations |
| 3 | 1213 | 14 | YES | angle deformations |
| 4 | 2582 | 0 | NO | bond stretches |
| 5 | 2715 | 126 | YES | bond stretches |
| 6 | 2715 | 126 | YES | bond stretches |
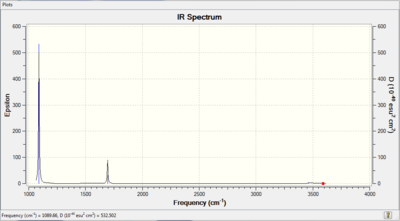
There are two sets of degenerate vibrations, one is a bond stretch and one is a bond angle deformation, and this would lead to two single peaks in the IR spectrum. Therefore, 3 peaks would appear on the spectrum.
MO Diagram of BH3
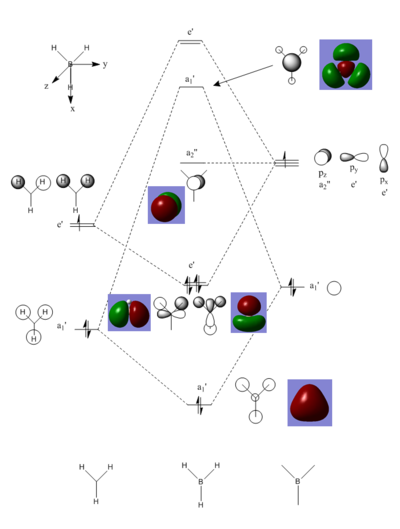
The real MOs and LCAO MOs are similar. The MO theory is accurate and useful.
Smf115 (talk) 08:44, 17 May 2018 (BST)Good inclusion of some of the MOs however, the top two are missing from the diagram and the comment could have mentioned some of the subtle differences between the MOs and the LCAOs.
NH3 molecule
B3LYP/6-31G level
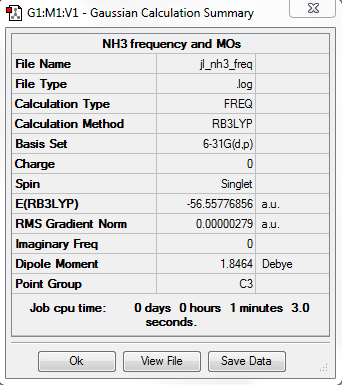
Item Table
Item Value Threshold Converged? Maximum Force 0.000005 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.000012 0.001800 YES RMS Displacement 0.000006 0.001200 YES
Frequency analysis log file: jl_nh3_freq.log
Low Frequency lines
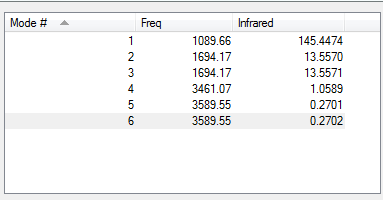
Low frequencies --- -11.6527 -11.6490 -0.0044 0.0333 0.1312 25.5724 Low frequencies --- 1089.6616 1694.1736 1694.1736
Optimised NH3 molecule |
IR of NH3 molecule
NH3BH3 molecule
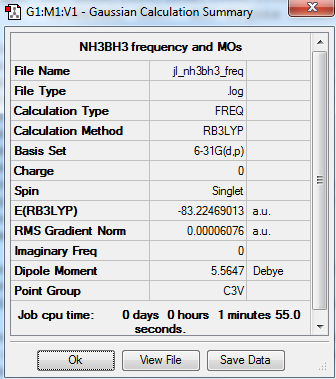
B3LYP/6-31G level
Item Table
Item Value Threshold Converged? Maximum Force 0.000118 0.000450 YES RMS Force 0.000061 0.000300 YES Maximum Displacement 0.000525 0.001800 YES RMS Displacement 0.000309 0.001200 YES
Frequency analysis log file: jl_nh3bh3_freq.log
Low Frequency lines
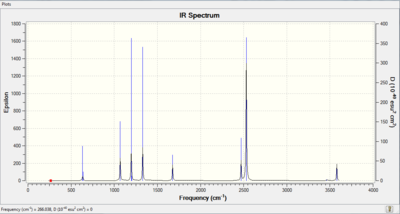
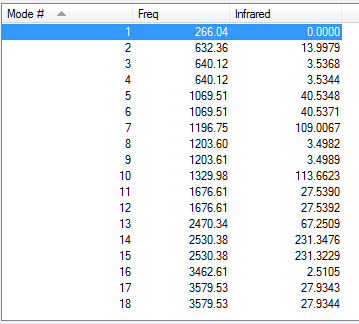
Low frequencies --- -0.0614 -0.0464 -0.0066 21.3656 21.3716 40.8051 Low frequencies --- 266.0519 632.3624 640.1162
Optimised NH3BH3 molecule |
IR of NH3BH3 molecule
Association energies: Ammonia-Borane
E(NH3)= -56.558 a.u. E(BH3)= -26.615 a.u. E(NH3BH3)= -83.225 a.u. ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]= -83.225-(-56.558-26.615)= -0.052a.u.= -136.5 kJ/mol The bond is weak, as the bond energy of C-C is 348 kJ/mol, so the N-B bond is weak.
BBr3 molecule
B3LYP/B 6-31G/Br LanL2DZ level
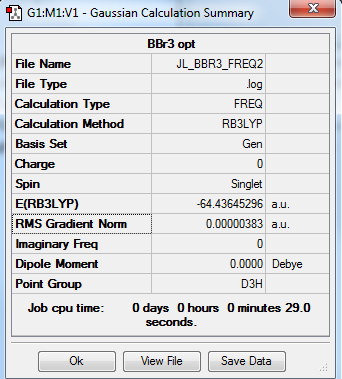
Item Table
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000036 0.001800 YES RMS Displacement 0.000018 0.001200 YES
Frequency analysis file: http://hdl.handle.net/10042/202334 and the DOI is DOI:10042/202334
Low Frequency lines
Low frequencies --- -0.0137 -0.0064 -0.0046 2.4315 2.4315 4.8421 Low frequencies --- 155.9631 155.9651 267.7052
Optimised BBr3 molecule |
IR of BBr3 molecule
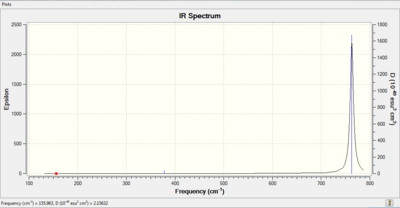
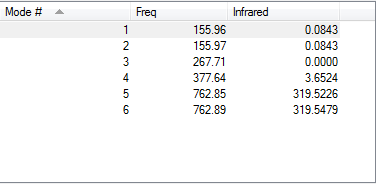
Smf115 (talk) 08:43, 17 May 2018 (BST)Nice inclusion of the vibrational spectrum and good recording of the calcualtion method used including the pseudopotential.
Project - Lewis acids and bases
Five possible isomers and the symmetry
Five possible isomers:
 |
 |
 |
 |
 |
Isomer A with 2 bridging Br ions
B3LYP/Al,Cl 6-31G/Br LanL2DZ level
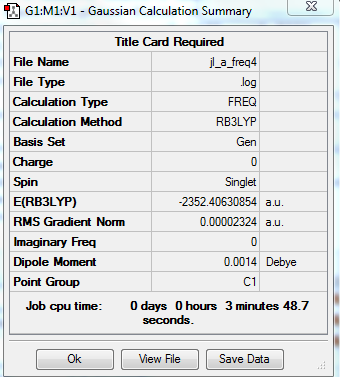
Item Table
Item Value Threshold Converged? Maximum Force 0.000067 0.000450 YES RMS Force 0.000023 0.000300 YES Maximum Displacement 0.001141 0.001800 YES RMS Displacement 0.000499 0.001200 YES
Frequency analysis log file: jl_a_freq4.log
Low Frequency lines
Low frequencies --- -5.1730 -4.9021 -3.0082 0.0013 0.0031 0.0035 Low frequencies --- 14.8117 63.2813 86.0726
Optimised Al2Cl4Br2 molecule |
Isomer B with trans terminal Br and bridging Cl ions
B3LYP/Al,Cl 6-31G/Br LanL2DZ level
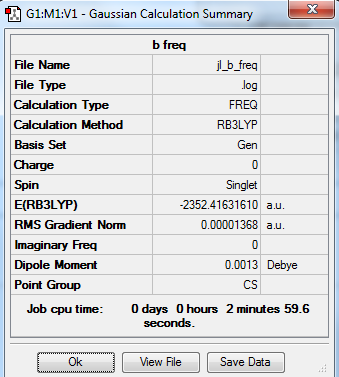
Item Table
Item Value Threshold Converged? Maximum Force 0.000081 0.000450 YES RMS Force 0.000042 0.000300 YES Maximum Displacement 0.001588 0.001800 YES RMS Displacement 0.000974 0.001200 YES
Frequency analysis log file: jl_b_freq.log
Low Frequency lines
Low frequencies --- -0.0035 -0.0022 0.0009 1.3568 3.6367 4.2604 Low frequencies --- 120.5042 133.9178 185.8950
Optimised Al2Cl4Br2 molecule |
Relative energy of Isomers A and B
E(A)= -2352.406 a.u. E(B)= -2352.416 a.u. Relative energy= -26 kJ/mol The isomer B is more stable as it has a lower energy. Br atom is bigger than Cl and bond length between Al and Br is 2.49Å. The bond length between Al and Cl is 2.30Å, which is shorter. Therefore isomer B is more stable.
Monomer AlCl2Br
B3LYP/Al,Cl 6-31G/Br LanL2DZ level
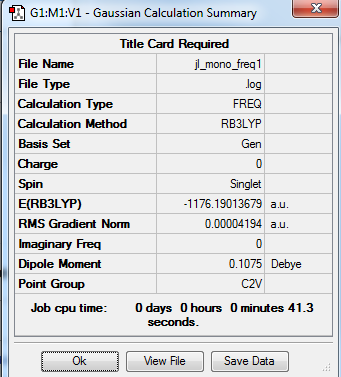
Item Table
Item Value Threshold Converged? Maximum Force 0.000067 0.000450 YES RMS Force 0.000023 0.000300 YES Maximum Displacement 0.001141 0.001800 YES RMS Displacement 0.000499 0.001200 YES
Frequency analysis log file: jl_mono_freq1.log
Low Frequency lines
Low frequencies --- -5.1730 -4.9021 -3.0082 0.0013 0.0031 0.0035 Low frequencies --- 14.8117 63.2813 86.0726
Optimised AlCl2Br molecule |
The dissociation energy for Isomer B into 2AlCl2Br
E(Al2Cl4Br2)= -2352.416 a.u. E(AlCl2Br)= -1176.190 a.u. 2xE(AlCl2Br)= -2352.380 a.u. ΔE=2xE(AlCl2Br)-E(Al2Cl4Br2)= -2352.380-(-2352.416)= 0.036a.u.= 95 kJ/mol The dissociation energy is a positive value, which means Al2Cl4Br2 is more stable than the monomer.
MOs of Al2Cl4Br2
There are 48 valence electrons, so 24 valence MOs.
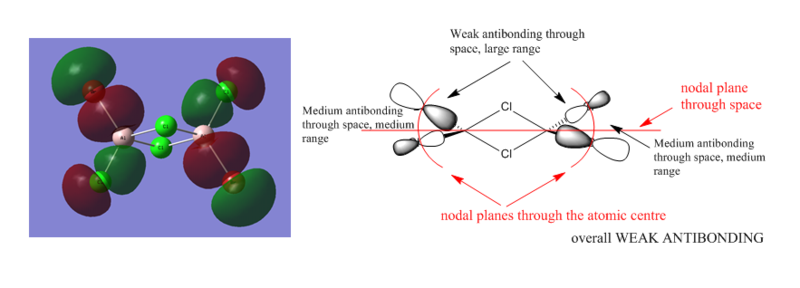
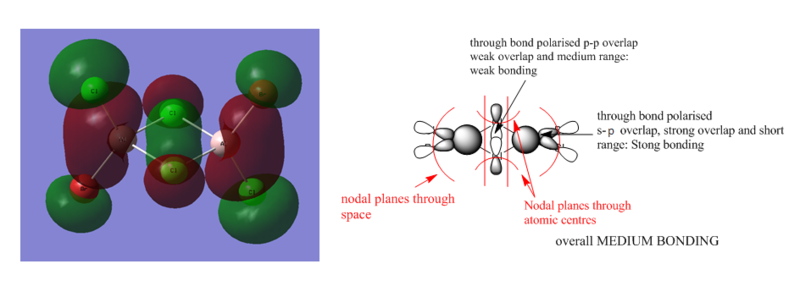
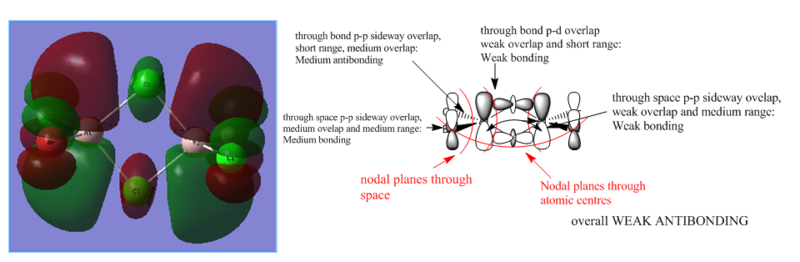
Smf115 (talk) 08:40, 17 May 2018 (BST)Clearly annoted LCAOs with identification of the nodal planes and the nature and strength of the main interactions. To improve, the LCAOs aren't fully correct for MO 57 with the contribution on the Cl atoms coming from the 4s orbitals and not d-orbitals. Great to see MO 57 chosen though!
Smf115 (talk) 08:45, 17 May 2018 (BST)Overall a solid report with a good project section. Mistakes were made throughout the first section though but well presented structure information throughout.
