Rep:Mod:JK718
NH3
[BIDROW]
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -56.55776873 a.u |
| RMS Gradient | 0.00000485 a.u |
| Point Group | C3V |
| Bond Length | 1.02 A |
| Bond Angle | 105.7 |
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
NH3 |
Display Vibrations
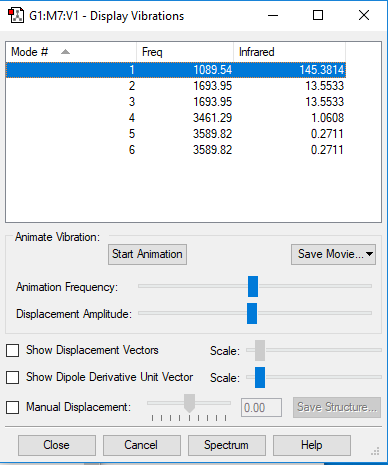
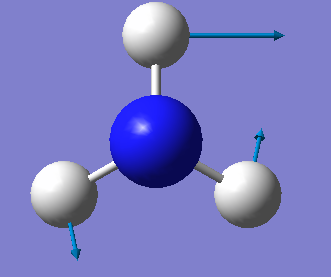
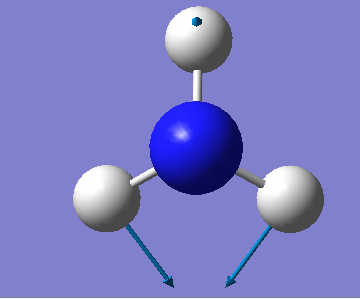
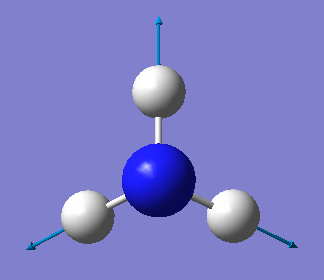
| Wavenumber/cm-1 | 1090 | 1694 | 1694 | 3461 | 3590 | 3590 |
| Symmetry | A1 | E | E | A1 | E | E |
| Intensity/ arbitrary units | 145 | 13.6 | 13,6 | 1.06 | 0.27 | 0.27 |
| Image | 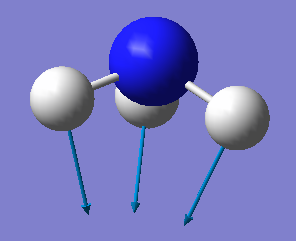 |
 |
 |
 |
 |
|
From the 3N-6 rule, we would expect 6 modes for an ammonia molecule whereby 2 of these modes are degenerate at 1693cm-1 and 3589-1. The modes at 1089cm-1, 3461cm-1 and 3589cm-1 are bond stretch vibrations while the modes at 1693cm-1 are bending vibrations. The mode at 3461cm-1 is highly symmetric. The umbrella mode is at 1089cm-1. In an experimental spectrum of gaseous ammonia, the number of bands seen would be 2.
Charge
| Charge on N atom | -1.125 |
| Charge on ̃H atom | 0.375 |
The charge expected on the nitrogen atom would be -1 due to the electronegativity of the atom. Therefore the charge expected on a hydrogen atom would be 0.333 for an overall neutral molecule.
N2
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -109.52412868 a.u |
| RMS Gradient | 0.00000060 a.u |
| Point Group | C3V |
| Bond Length | 1.11 A |
| Bond Angle | None |
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
N2 |
Display Vibrations
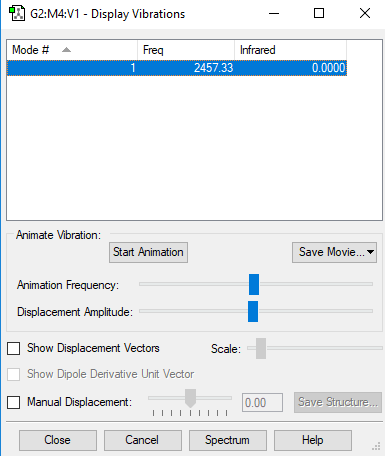
| Wavenumber/cm-1 | 2457 |
| Symmetry | SGG |
| Intensity/ arbitrary units | 0.00 |
| Image | 
|
Charge
| Charge on N atom | 0.00 |
H2
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -1.17853936 a.u |
| RMS Gradient | 0.00000017 a.u |
| Point Group | DinfH |
| Bond Length | 0.74 A |
| Bond Angle | None |
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.164080D-13
Optimization completed.
-- Stationary point found.
H2 |
Display Vibrations
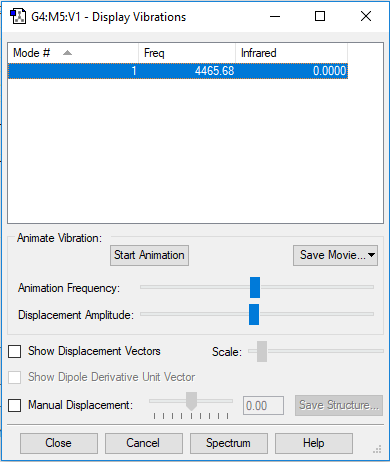
| Wavenumber/cm-1 | 4465 |
| Symmetry | SGG |
| Intensity/ arbitrary units | 0.00 |
| Image | 
|
| Charge on H atom | 0.00 |
Transition Metal Complexː BIDROW
[BIDROW]
| Normal H-H Bond Length | 0.740 A |
| BIDROW H-H Bond Length | 0.995 A |
The transition metal hydrogen bond distance is longer than the usual hydrogen bond length. This indicates a weaker bond. Given that bond strength is determined by the even distribution of electron density between 2 atoms, it goes to show that when a transition metal is attached to a hydrogen, it attracts the electron density from the neighbouring H-H bond and as a result strengthens the transition metal hydrogen bond and weakens the H-H bond resulting in a longer bond length. Computational error must also be accounted for when measuring bond lengths which may also cause differences in values.
I also found the crystal structure of BIDROWː
Haber-Bosch Process
| E(NH3) | -56.55776873 a.u |
| 2*E(NH3) | -113.1155375 a.u |
| E(H2) | -1.17853936 a.u |
| E(N2) | -109.5241287 a.u |
| 3̈E(H2) | -3.53561808 a.u |
| 2*E(NH3)-[E(N2)+3*E(H2)] | -0.0557907 a.u |
| 2*E(NH3)-[E(N2)+3*E(H2)]/kJ/mol-1 | -146.4784829 |
The product is more stable than the reatcants as the change in energy is negative.
NF3
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -354.07131058 a.u |
| RMS Gradient | 0.00010256 a.u |
| Point Group | C3V |
| Bond Length | 1.38 A |
| Bond Angle | 101.8 |
Item Value Threshold Converged?
Maximum Force 0.000164 0.000450 YES
RMS Force 0.000108 0.000300 YES
Maximum Displacement 0.000612 0.001800 YES
RMS Displacement 0.000296 0.001200 YES
Predicted change in Energy=-1.274066D-07
Optimization completed.
-- Stationary point found
NF3 |
Display Vibrations
| Wavenumber/cm-1 | 4465 |
| Symmetry | SGG |
| Intensity/ arbitrary units | 0.00 |
| Image | 
|
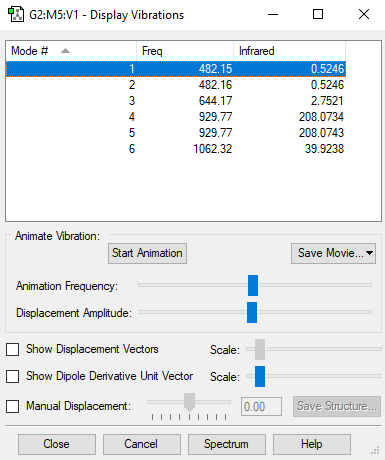
| Wavenumber/cm-1 | 482 | 482 | 644 | 930 | 930 | 1062 |
| Symmetry | A1 | E | E | A1 | E | E |
| Intensity/ arbitrary units | 0.52 | 0.52 | 2.75 | 208 | 208 | 39.9 |
| Image |  |
 |
 |
 |
 |
|
Charge
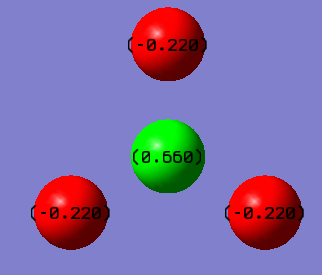
| Charge on N atom | 0.550 |
| Charge on ̃F atom | -0.220 |
Molecular Orbitals
Marking
Note: All grades and comments are provisional and subjecct to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have recieved your grade from blackboard.
Wiki structure and presentation 1/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES
Do you effectively use tables, figures and subheadings to communicate your work?
YES
NH3 0/1
Have you completed the calculation and given a link to the file?
NO - You missed to include a link to the .log file of your finished calculation. This reduces the achievable mark for this section by 1.
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES - You answered all questions correctly!
N2 and H2 0/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
NO - You missed to include a link to the .log file of your finished calculation. This reduces the achievable mark for this section by 1. You could have explained that the charges are 0 as the electronegativities are equal.
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES
Have you compared your optimised bond distance to the crystal structure bond distance?
YES
Haber-Bosch reaction energy calculation 0.5/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
YES
Have you reported your answers to the correct number of decimal places?
NO - the energy in kJ/mol should only be reported to one decimal place!
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES
Your choice of small molecule 2.5/5
Have you completed the calculation and included all relevant information?
YES
Have you added information about MOs and charges on atoms?
NO - You missed to include a link to the .log file of your finished calculation. This reduces the achievable mark for this section by 1. You could have explained that the calculated charges with an electronegativity argument. You should have explained the calculated vibrations modes (which are degenerate, how many modes you expected, how many band are expected in an experimental spectrum....) You missed to give the AOs contributing to the MOs in 3 cases. However for the ones you are giving the AOs these are correct. As well you missed to describe the contribution to bonding for some of the MOs (MO 1 and 6). Additionally you were asked to comment on if the MOs are occupied/unoccupied.
Independence 0/1
If you have finished everything else and have spare time in the lab you could: Check one of your results against the literature, or Do an extra calculation on another small molecule, or Do some deeper analysis on your results so far
NO - No independent work has been identified.