Rep:Mod:JK21055
Part 6: Structural properties and the radial distribution function
Task 1
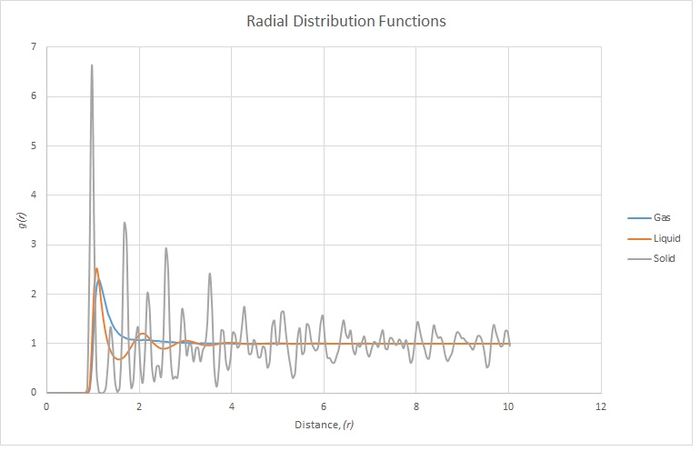
The three different phases show very different distributions. All three show a very low probability until a distance of r=1. This is due to the Lennard-Jones potential being very high. All three phases also show a peak at 1 which is the equilibrium energy, so it is most favourable for neighbours to sit here.
The solid is in a face centred cubic lattice which means that an atom's neighbours are going to be very particular distances away. This explains why there are sudden peaks and troughs in probability.
The liquid is more diffuse and fits a weak, almost lattice-like, pattern while the Lennard Jones potential is favourable. Further away, the distribution becomes random. It is equally likely to find an atom at any distance at high (r).
The gas is very disordered and is distributed completely randomly, though the probability is higher while there is a favourable Lennard Jones potential. The average probability tends to 1 as the Lennard-Jones potential tends to 0.
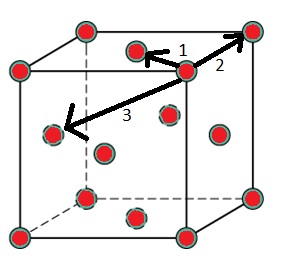
Figure 2 illustrates which are the closest three lattice positions to the central atom. These are therefore the three first peaks in the RDF graph.
The lattice spacing equals 1.375, the x-value of the second peak on the RDF graph, as this is the second closest position to the central atom.
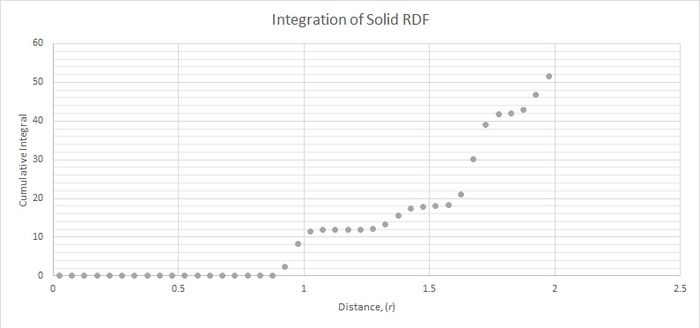
Figure 3 was used to calculate the coordination number for the first three peaks. The central atom coordinates to the closest position (marked 1 in figure 2) 12 times. It coordinates to the second closest position 6 times and to the third closest position 24 times.
