Rep:Mod:JK21053
Part 4: Running Simulations under Specific Conditions
Task 1
Determining .
We are given:
Substitute for and then substitute for to give:
Solve these two equation simultaneously to give:
and therefore:
Task 2
It is easier to explain the numbers in reverse order. The third number dictates how often (in terms of timesteps) to take an average. The second number controls how many input values will be used in the average. The first number controls how far apart the input values will be in the average. In this particular simulation, the average is taken every 100,000 timesteps and 1,000 values contribute to each average, with the input values being found 100 timesteps apart.
This simulation will run for 100,000 timesteps.
Task 3
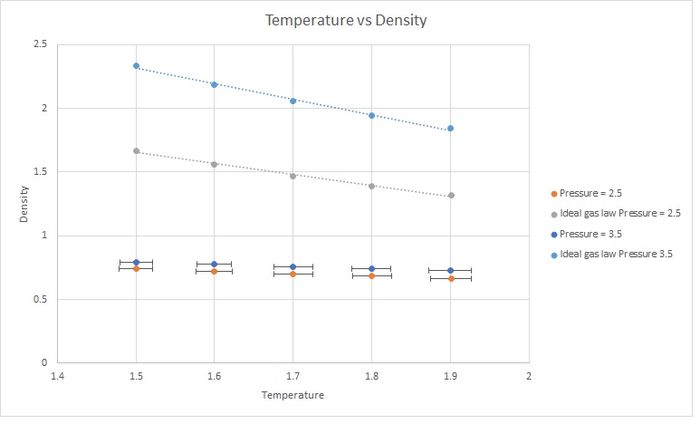
The simulated density is lower. This is because the Ideal Gas Law does not take into account interactions between atoms. Therefore they can get much closer to each other at the same pressure, increasing the density. The simulation considers interactions (as it uses the Lennard-Jones potential), preventing atoms from becoming so close because the potential energy is too high. The discrepancy increases with pressure.
