Rep:Mod:JEL3117
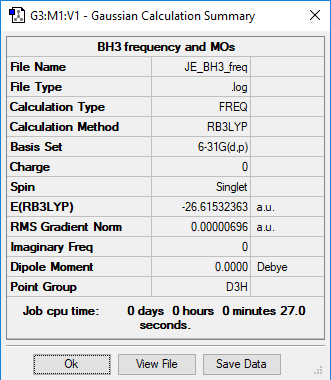
Analysing a BH3 molecule
RB3LYP/6-31G(p,d) level
Summary table of BH3
Item Value Threshold Converged? Maximum Force 0.000014 0.000450 YES RMS Force 0.000007 0.000300 YES Maximum Displacement 0.000055 0.001800 YES RMS Displacement 0.000027 0.001200 YES
Frequency analysis log file Media:JE_BH3_FREQ.LOG
Low frequencies --- -7.9073 -1.6385 -0.0054 0.6256 6.5697 6.7709 Low frequencies --- 1162.9662 1213.1623 1213.1650
Optimised BH3 molecule |
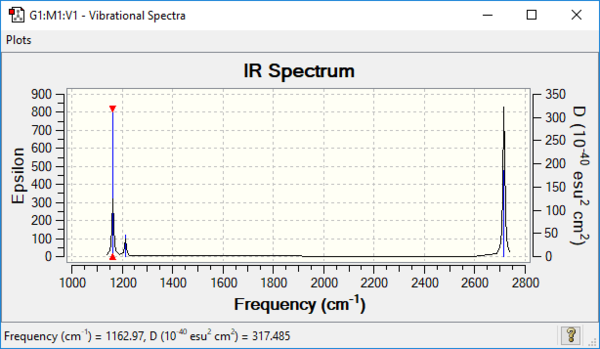
Vibrational spectrum for BH3
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1162 | 93 | A2 | yes | out-of-plane bend |
| 1213 | 14 | E' | very slight | bend |
| 1213 | 14 | E' | very slight | bend |
| 2582 | 0 | A1' | no | symmetric stretch |
| 2716 | 126 | E' | yes | asymmetric stretch |
| 2716 | 126 | E' | yes | asymmetric stretch |
Q): why are there fewer vibrational peaks than there are vibrations
A): Based on the number of modes of vibration, we are expected to see 6 peaks on IR. However, Molecules are IR active if there is a change in dipole moment in the molecule. The symmetric stretch at 2582cm-1 has no change in dipole moment and therefore it is not IR active. The degeneracies are observed for asymmetric stretch at 2716cm-1 and bend at 1213cm-1. The degenerate orbitals are shown as one peak. As a result, only 3 peaks are shown on the IR diagram.
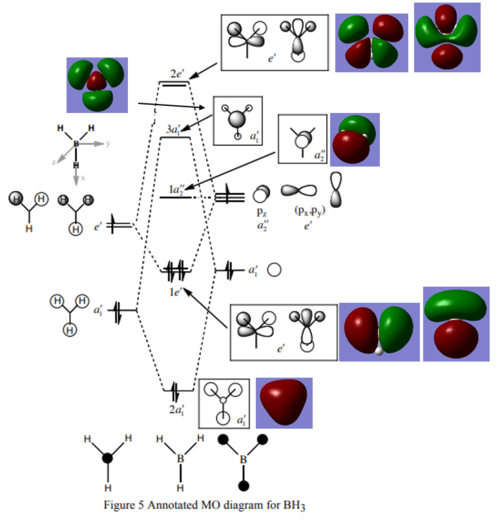
Q): Are there any significant differences between the real and LCAO MOs?
A): For the real MOs, the orbitals with the same phases overlap and the orbitals with opposite phases cancel out while LCAO MOs show individual MOs.
Q): What does this say about the accuracy and the usefulness of qualitative Mos?
A): Although qualitative Mos are not a perfect representation of the real MOs but the analysis of qualitative MOs provides a good prediction of the real Mos . As a result, the qualitative MOs are relatively useful and accurate in predicting the real MOs.
Smf115 Clear representation of all of the relevant MOs on the diagram and evaluation of the usefulness of the LCAO approach. The difference between the real and LCAO MOs highlighted isn’t correct though. When you construct your LCAO MOs from the atomic orbitals then you do use in-phase addition and out of phase cancellation, particularly when assessing the relative contribution of each AO to the final MO. Although the MOs are still drawn in terms of the AO shapes the final shape is comparable across to the real ones.
Association energies : Ammonia-Borane
E(NH3) = -56.55776873 hartree E(BH3) = -26.61532363 hartree E(NH3BH3) = -83.22469032 hartree
ΔE =E(NH3BH3)-[E(NH3)+E(BH3)]
=-83.22469032-(-56.55776873-26.6153263) =-0.05159796 hartree =-135 kJ/mol = 135 kj/mol
It is a sensible bond energy value because it is in the range of the hundreds of kj/mol. However, in comparison with the literature value which is 377.9 ± 8.7 kj/mol, the calculated B-N bond strength is weaker. This shows that the B-N dative bond is a weaker than the normal B-N bond.
Correct calculation and good consideration of the accuracy of the resulting association energy calculated. You have used a sensible comparison which is great, however, your literature value lacks a reference and to improve you could consider some other bond dissociation energies to fully evaluate the strength of the bond. You are also missing any sections on your NH3 and NH3BH3 molecules themselves! The optimisation/frequency information and the log files should have been present for both structures. Smf115 (talk) 23:16, 12 May 2019 (BST)
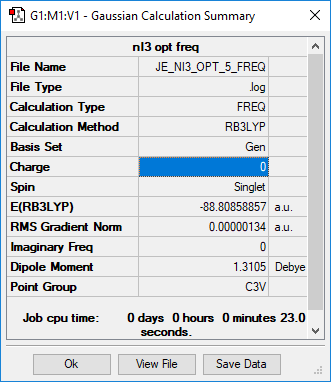
Day 1 : Analysis of NI3
RB3LYP/6-31G(p,d) level
Summary of NI3
Item Value Threshold Converged? Maximum Force 0.000002 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000023 0.001800 YES RMS Displacement 0.000013 0.001200 YES
Frequency analysis log file Media:JE_NI3_FREQ.LOG
Low frequencies --- -12.5522 -12.5460 -6.0047 -0.0039 0.0191 0.0664 Low frequencies --- 100.9969 100.9977 147.3377
Optimised BH3 molecule |
Length of optimised N-I distance = 2.18396 Å
Day 2 and 3: Lewis acids and bases Projects
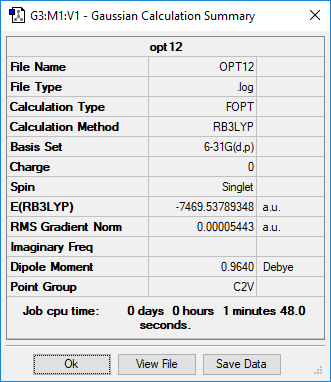
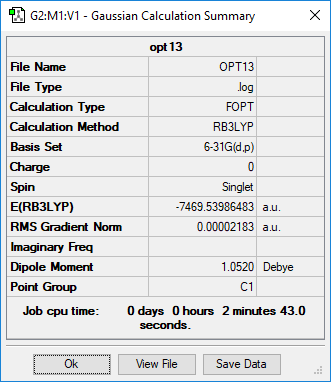
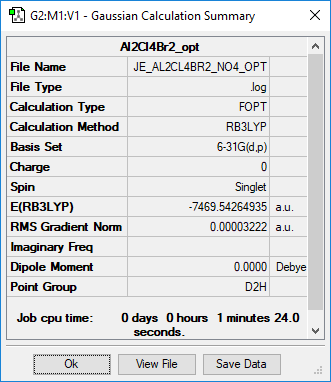
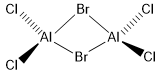
Summary page of AlCl4Br2 isomers
RB3LYP/6-31G(p,d) level
Summary page of Isomer 1
Summary page of Isomer 2
Summary page of Isomer 3
Summary page of Isomer 4
Summary page of Isomer 5
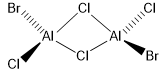
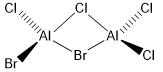
Five possible isomers and the symmetry of each isomer of Al2Cl4Br2
Energy of the isomers with 2 bridging Br ions (isomer 4) and the isomer with trans terminal Br and bridging Cl ions (isomer 2)
Energy of isomer 4 = -7469.54264935 hartree
Energy of isomer 2 = -7469.53758218 hartree
Relative energies of the isomers = energy of isomer 4 - energy of isomer 2
= -7469.5426935 - (-7469.53758218)
= -0.00511132 hartree
= -13.419 kJ/mol
= -13 kJ/mol
The relative stability of these conformers with respect to the bridging ions
The energy of isomer 4 is lower that the energy of isomer 2 by 13 kJ/mol. It shows that isomer 4 is a more stable conformer than isomer 2.
The dissociation energy for the lowest energy conformer into 2AlCl2Br
Isomer 4 has the lowest energy by looking at the summary table above.
The dissociation energy = energy of isomer 4 - 2(energy of AlCl2Br)
= -7469.54264935 - 2(-3734.74850528)
= -0.04563879 hartree
= -119.824 kJ/mol
= -120 kJ/mol
Since the product (isomer 4) has a lower energy than 2AlCl2Br by 120 kJ/mol, the product is more stable than the isolated monomers.
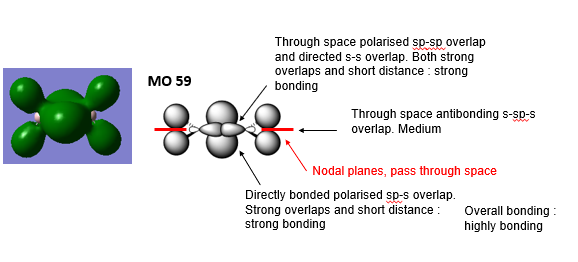
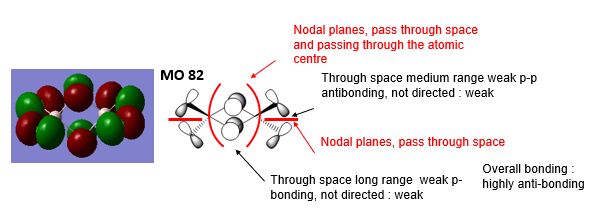
3 MOs ranging from highly bonding to highly antibonding
Highly Bonding MO 59
Weakly Bonding MO 72
Highly Bonding MO 82
Good attempt at describing the main interactions, a good range of MOs was selected and the overall character of the MOs has been evaluated well, except MO82 is more likely to be weak antibonding as the antibonding interactions are quite distant. To improve, the labelling of the interactions and nodal planes could be clearer and the description of the interactions generally doesn’t use the correct or clear terminology, e.g. ‘directly bonded polarised’ and could be more precise. Smf115 (talk) 23:18, 12 May 2019 (BST)
Overall, an ok attempt at the lab and the analysis parts completed have been done well. Your report has been let down by lacking a lot of content, particularly the structures and information for both sections. Smf115 (talk) 23:18, 12 May 2019 (BST)
Reference
- ↑ Patricia Hunt (2019) Lecture 4 Tutorial Problem Model Answer. [Workshop] Imperial College London, 2nd May