Rep:Mod:JA97
NH3 Molecule
Questions on the procedure taken and the results from the calculation
The molecule NH3 was created using the program GaussView and then calculations were run on the molecule in order to get the optimised molecule where the bond angles and bond lengths are optimised to produce the lowest energy version of the molecule. The calculation method used was RB3LYP and the basis set used was 6-31G(dp). The final energy of the molecule was calculated to be -56.55776873 Au (atomic units) and the RMS gradient calculated was 0.00000485. The point group of the NH3 is C3V. The calculations run produced an optimised N-H bond length of 1.01798 Å and optimised H-N-H bond angle of 105.74°
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986286D-10
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.018 -DE/DX = 0.0 !
! R2 R(1,3) 1.018 -DE/DX = 0.0 !
! R3 R(1,4) 1.018 -DE/DX = 0.0 !
! A1 A(2,1,3) 105.7412 -DE/DX = 0.0 !
! A2 A(2,1,4) 105.7412 -DE/DX = 0.0 !
! A3 A(3,1,4) 105.7412 -DE/DX = 0.0 !
! D1 D(2,1,4,3) -111.8571 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
NH3 molecule |
The optimisation file is liked to here
Vibrations of the NH3 molecule
6 modes are expected from the 3N-6 rule (3 * 4 - 6). Modes 2 and 3 are degenerate. Modes 5 and 6 are degenerate. This can be seen as they have the same frequencies. Bending vibrations: Mode 1, 2 and 3. Stretching vibrations: Modes 4, 5 and 6. The highly symmetric mode is Mode 4. The mode which is known as the "umbrella mode" is Mode 1. 2 bands would be expected in an experimental spectrum of gaseous ammonia run in an infrared spectrometer. This is because only modes 1, 2 and 3 have intensities high enough to be seen in the spectrum. Only two appear because modes 2 and 3 have the same frequencies and hence wave numbers. Modes 4, 5 and 6 do not appear in the spectrum because the change in dipole is not big enough to be seen in the spectrum.
A gif of each of the vibrations for the NH3 molecule can be found here vibration 1 vibration 2 vibration 3 vibration 4 vibration 5 vibration 6
Haber process calculation
The energy for the following reaction: N2 + 3H2 -> 2NH3 was calculated using the equation below.
ΔE=2*E(NH3)-[E(N2)+3*E(H2)] * 2625.5 = -146.48 kj/mol
(2*-56.55776873) - [(-109.52412868) + (3 * -1.17853935)] * 2625.5 = -146.48 kj/mol
H2 molecule
H2 molecule |
The optimisation file is liked to here
Item Value Threshold Converged?
Maximum Force 0.000066 0.000450 YES
RMS Force 0.000066 0.000300 YES
Maximum Displacement 0.000086 0.001800 YES
RMS Displacement 0.000123 0.001200 YES
Predicted change in Energy=-5.726835D-09
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 0.7429 -DE/DX = -0.0001 !
Bond length is 0.74289 Å and the bond angle is 180°.
N2 molecule
N2 molecule |
The optimisation file is liked to here
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000001 0.001800 YES
RMS Displacement 0.000002 0.001200 YES
Predicted change in Energy=-4.428711D-12
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1055 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
Bond length is 1.10550 Å and the bond angle is 180°. Gaussview does not allow the bond angles to be calculated for diatomic molecules and so since the structure produced is linear, the bond angle can be assumed to be 180°. However this may deviate from reality due to lone pairs distorting the bond angles.
Charges of atoms of NH3
The charge on the nitrogen is -1.125
The charge on the hydrogen atom is 0.375
Nitrogen is a more electronegative element than hydrogen because it has more protons in its nucleus while still having a small atomic radius. As a result, the electrons shared between the atoms experience a bigger nuclear charge from the nitrogen atom which pulls the electrons towards itself. Therefore there is a higher electron density around the nitrogen atom than the hydrogen atoms and so the charge on the nitrogen atom is expected to be negative and the charge on the hydrogen is expected to be positive.
Project molecule - H2O
Questions on the procedure taken and the results from the calculation
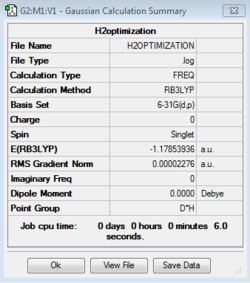
The molecule H2O was created using the program GaussView and then calculations were run on the molecule in order to get the optimised molecule where the bond angles and bond lengths are optimised to produce the lowest energy version of the molecule. The calculation method used was RB3LYP and the basis set used was 6-31G(dp). The final energy of the molecule was calculated to be -76.41973740 Au (atomic units) and the RMS gradient calculated was 0.00006276. The point group of a H2O molecule is C2V. The calculations run produced an optimised O-H bond length of 0.96522 Å and optimised O-H-O bond angle of 103.75°
Item Value Threshold Converged? Maximum Force 0.000099 0.000450 YES RMS Force 0.000081 0.000300 YES Maximum Displacement 0.000115 0.001800 YES RMS Displacement 0.000120 0.001200 YES Predicted change in Energy=-1.939669D-08 Optimization completed. Stationary point found ! Optimized Parameters ! ! (Angstroms and Degrees) ! -------------------------- -------------------------- ! Name Definition Value Derivative Info. ! -------------------------------------------------------------------------------- ! R1 R(1,2) 0.9652 -DE/DX = 0.0001 ! ! R2 R(1,3) 0.9652 -DE/DX = 0.0001 ! ! A1 A(2,1,3) 103.7454 -DE/DX = 0.0 ! --------------------------------------------------------------------------------
H2O molecule |
The optimisation file is liked to here
O2 molecule
H2O molecule |
The optimisation file is liked to here
Item Value Threshold Converged?
Maximum Force 0.000002 0.000450 YES
RMS Force 0.000002 0.000300 YES
Maximum Displacement 0.000001 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.793550D-12
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.2159 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
Bond length is 1.21594 Å and the bond angle is 180°. Gaussview does not allow the bond angles to be calculated for diatomic molecules and so since the structure produced is linear, the bond angle can be assumed to be 180°. However this may deviate from reality due to lone pairs distorting the bond angles.
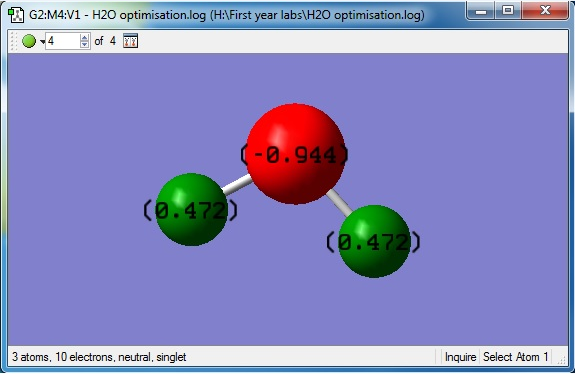
Charges of the atoms in H2O
The charge on the oxygen atom is -0.944
The charge on the hydrogen atom is 0.472
Oxygen is a more electronegative element than hydrogen because it contains more protons while still having a small atomic radius and so the shared electrons will experience a bigger nuclear charge from the oxygen atom in the shared bond. As a result this will attract the electrons more towards the oxygen atom and so the electron density around the oxygen atom will be higher. As a result, the charge on the oxygen atom is expected to be negative and the charge on the hydrogen atoms is expected to be positive.
Energies for the formation of H2O from its constituents elements
The energy for the following reaction: 1/2O2 + H2 -> H2O was calculated using the equation below.
ΔE=E(H2O)-[E(H2)+1/2*E(O2)] * 2625.5 = -295.33 kj/mol
(-76.41973740) - [(-1.17853935) + (1/2 * -150.25742435)] * 2625.5 = -295.33 kj/mol
The literature value found for the enthalpy of formation of H2O is: -286.629 kJ/mol [1]. The calculated value is quite close to the literature value found however the difference is due to the fact that the program only works out the energy change for one molecule of H2O while the literature value takes into account several molecules of H2O. There will be no hydrogen bonding in the calculation run as there is only one H2O molecule.
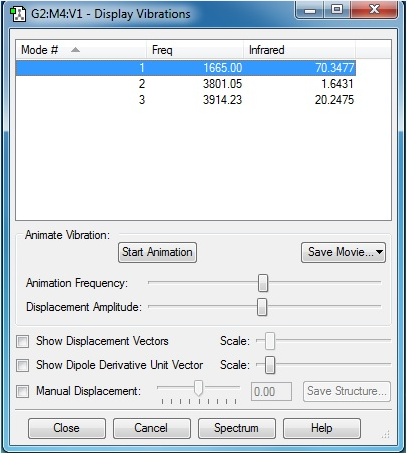
Vibrations of H2O molecule
3 modes are expected from the 3N-6 rule. Vibration 1 is a bending vibration and vibrations 2 and 3 are bond stretch vibrations. Three peaks are expected if a H2O molecule is run under an infrared spectrometer as none of them are degenerate in energy. Mode 2 is symmetrical.
A gif of the three vibrations of the H2O molecule can be found here: Vibration 1 Vibration 2 Vibration 3
Orbitals of H2O
This picture shows a σ bonding orbital which was made up of the 2S orbital from the oxygen atom and the 1S orbital from the hydrogen atom. It is a highly symmetric bonding orbital. It has a large energy of: -0.99736. The molecular orbital is occupied with electrons and so a bond will form. This orbital is the lowest in energy from the 5 chosen because it is formed from the 2S orbital of the oxygen which is more penetrating than the 2p orbital of the oxygen. As a result, it is closer to the nucleus of the oxygen atom and so experiences more nuclear charge and hence is more stable. Furthermore, in this orbital there is no nodal planes and there is complete overlap which results in a much stronger bonding orbital. The molecular orbital is deep in energy
This picture shows a σ bonding orbital which was made up of the 2P orbital from the oxygen atom and the 1S orbital from the hydrogen atom. It has an energy of: -0.51503. The molecular orbital is occupied with electrons and so a bond will form. The Molecular orbital is deep in energy.
This picture shows a σ* anti bonding orbital which was made up of the 2S orbital from the oxygen atom and the 1S orbital from the hydrogen atom. It has an energy of -0.37102. The molecular orbital is occupied with electrons which will therefore break the σ bond created from the 2S atomic orbital of the oxygen and the 1S atomic orbital of the hydrogen. The Molecular orbital is deep in energy.
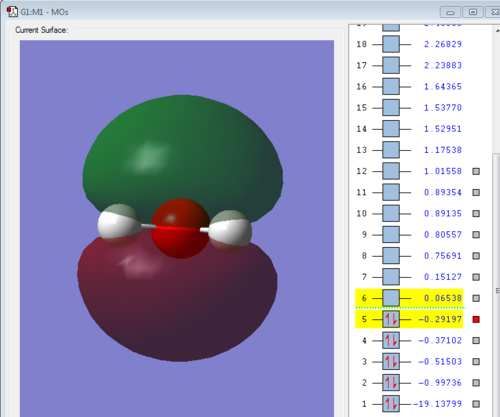
This picture shows a filled 2P atomic orbital. It has a medium sized energy of: -0.29197. This orbital is the HOMO which can form a bond with other molecules.
This picture shows a σ* anti bonding orbital which was made up of the 2P orbital from the oxygen atom and the 1S orbital from the hydrogen atom. It has an energy of: 0.15127. The molecular orbital is not occupied with electrons and so the bond created between the 2P orbital from the oxygen atom and the 1S orbital from the hydrogen atom remains. The molecular orbital is high in energy.
References
1. B. Ruscic, Active thermochemical tables: water and water dimer, J. Phys. Chem. A 117 (2013) 11940-11953