Rep:Mod:InorganComp
Day1
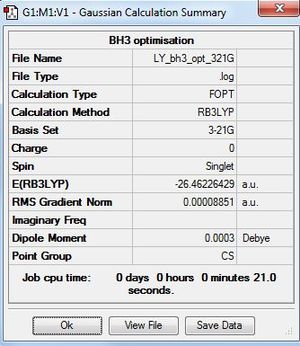
BH3:B3LYP/3-21G level
Optimisation log file here
Day2
BH3:B3LYP/6-31G-dp level
Optimisation log file here
| summary data | convergence | Jmol | |||
|---|---|---|---|---|---|

|
Item Value Threshold Converged? Maximum Force 0.000012 0.000450 YES RMS Force 0.000008 0.000300 YES Maximum Displacement 0.000063 0.001800 YES RMS Displacement 0.000039 0.001200 YES |
|
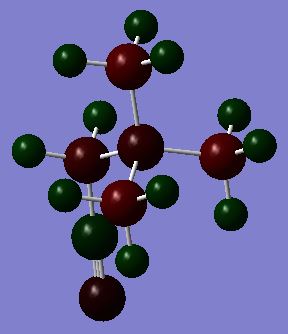
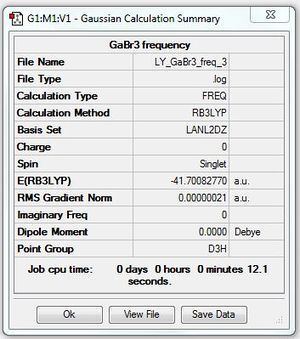
GaBr3:B3LYP/LANL2DZ level
optimisation file: DOI:10042/87417
| summary data | convergence | Jmol | |||
|---|---|---|---|---|---|

|
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000003 0.001800 YES
RMS Displacement 0.000002 0.001200 YES
Predicted change in Energy=-1.307748D-12
Optimization completed.
-- Stationary point found.
|
|
BBr3:B3LYP/Gen level
optimisation file: DOI:10042/87467
| summary data | convergence | Jmol | |||
|---|---|---|---|---|---|

|
Item Value Threshold Converged?
Maximum Force 0.000008 0.000450 YES
RMS Force 0.000005 0.000300 YES
Maximum Displacement 0.000035 0.001800 YES
RMS Displacement 0.000024 0.001200 YES
Predicted change in Energy=-4.142489D-10
Optimization completed.
-- Stationary point found.
|
|
EX3 Geometry Comparison Section
| BH3 | BBr3 | GaBr3 | |
|---|---|---|---|
| r(E-X) Å | 1.19 | 1.93 | 2.35 |
| θ(X-E-X) degrees(º) | 120.0 | 120.0 | 120.0 |
Changing ligand from H to Br for BX3, we see a decrease in bond length which means a decrease in bond energy. This is expected due to poorer overlap between the valence orbital of Br with B than H with B. Both H and Br need one more electron to complete their valence shell, however Br is much larger than H and Br has a larger electronegativity due to a more positively charged nucleus. The steric hinderance also increase the bond length of B-Br.
Changing the central atom from B to Ga increases the bond length of of E-X. B and Ga are both in group 3, therefore they have the same valence shell electronic configuration. However, Ga is much larger than B as it is further down the group and hence larger steric hindrance.
A bond is a favourable interaction between atoms or group of atoms and the electron density around them that leads to the formation of a stable chemical species.[1]
An ionic bond is generally considered as a strong bond. For example, LiF has a lattice enthalpy of 1030 KJ/mol, which is among the highest lattice enthalpy of ionic compounds formed by two signally charged ions.Single covalent bonds formed by elements from first three row of the periodic table are generally medium bonds. For example, C-C bond has a bond enthalpy of 348 KJ/mol and O-H bond has a bond enthalpy of 464 KJ/molHydrogen bonds and other intermolecular interactions are often considered as weak bonds. The strength of hydrogen bond in H3O is 22 KJ/mol.[2]
Gaussview recognises a bond only if the distance between the two atoms is smaller than a preset value. These values are based on organic molecules, whereas inorganic molecules often have longer bonds.
Day 3
BH3:B3LYP/6-31G(d,p) level
Frequency file: here
| summary data | low modes |
|---|---|

|
Low frequencies --- -12.4451 -12.4386 -7.8187 -0.0004 0.0235 0.4030 Low frequencies --- 1162.9687 1213.1351 1213.1353 |
Vibrational spectrum for BH3
| wavenumber (cm-1) | Intensity | IR active? | type |
| 1163 | 93 | yes | bend |
| 1213 | 14 | very slight | bend |
| 1213 | 14 | very slight | bend |
| 2582 | 0 | no | stretch |
| 2716 | 126 | yes | stretch |
| 2716 | 126 | yes | stretch |
Despite the fact that there are six vibrational modes present for the BH3 molecule, the two vibrational modes at 1213 cm-1 are equivalent to each other which in turn gives only one peak on the spectrum. The two vibrational modes at 2716 cm-1 are also equivalent and they give the second peak on the spectrum. The vibrational mode at 2582 cm-1 is IR inactive as it does not involve a change in dipole moment, whereas the vibrational mode at 1163 cm-1 is IR active and gives the third peak on the spectrum.
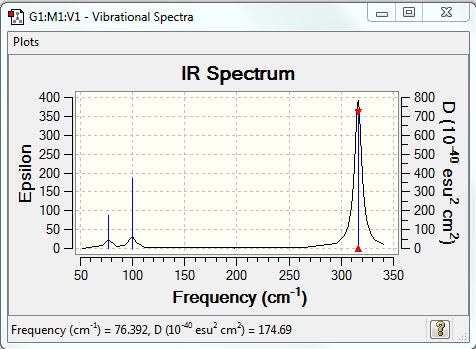
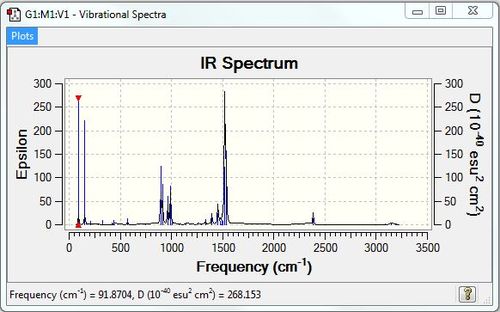
GaBr3:B3LYP/LANL2DZ level
Frequency file: DOI:10042/90519
| summary data | low modes |
|---|---|
|
Low frequencies --- -1.4877 -0.0015 -0.0003 0.0096 0.6540 0.6540 Low frequencies --- 76.3920 76.3924 99.6767 |
Vibrational spectrum for GaBr3
| wavenumber (cm-1) | Intensity | IR active? | type |
| 76 | 3 | very slight | bend |
| 76 | 3 | very slight | bend |
| 100 | 9 | very slight | bend |
| 197 | 0 | no | stretch |
| 316 | 57 | yes | stretch |
| 316 | 57 | yes | stretch |
compare and contrast
Large difference in vibrational frequencies
All the vibrational frequencies of the GaBr3 molecules are much lower than those of the BH3 molecules, which indicates that less energy is required to stretch or bend GaBr3 molecules than BH3 molecules. Therefore, the vibrational frequency data suggests that Ga-Br bond is weaker than the B-H bond, this is in good agreement with the bond length of these two molecules(B-H 1.19 vs. Ga-Br 2.35). This is expected as the valence orbitals of Ga and Br are more diffuse than that of B and H, resulting in poor overlap and hence weaker bond. Moreover, vibrational frequency is inversely proportional to the square root of the reduced mass. As Ga and Br are much heavier atoms than B and H, the vibrational frequencies should consequently be smaller for GaBr3 molecules.
Reordering of the modes
By looking at the vibrational modes of GaBr3 and BH3 in Gauss view, it is clear to identify that both of them contain two types of vibrational modes: one is the umbrella bending motion ( 1163 cm-1 for BH3 and 100 cm-1 for GaBr3) another one is a pair of equivalent twisted scissoring bending motions( 1213 cm-1 for BH3 and 76 cm-1 for GaBr3). The umbrella motion requires out of plane vibrations of either the central atom or the terminal atoms, where as the twisted scissoring motion requires in-plane vibration of both central and terminal atoms
For BH3, the terminal H atoms are lighter than the central B atom, therefore, vibrating all of the H atoms requires less energy than vibrating central B atoms and two of the terminal H atoms, which positions the umbrella motion at a lower frequency. From the displacement vectors, we can also see that this motion is dominated by the H atoms.
For GaBr3, the central Ga atom is only slightly lighter than the terminal Br atoms, which makes the umbrella motion less favorable due to the requirement of large change in momentum. Therefore, the umbrella motion for GaBr3 is at a higher frequency.
Answer to the questions
The computation method and basis set determine the potential energy surface generated for a molecule. The geometry optimisation locates the minimum on the potential energy surface, where as the frequency calculation is based on the second derivative around the minimum point. If the optimisation and frequency analysis are calculated based on different surface, then they are not directly related and there is no point of comparing them.
Frequency analysis can be used to determine whether the stationary point found by the optimisation is a minimum or not. If there are any negative frequencies appearing, it means that the molecule is at a saddle point or transition state rather than a minimum.
Low frequencies represent the translational and rotational motions of the center of mass of the molecule, which is approximately the same as the center of mass of the nucleus as they are much heavier. Ideally we want the low frequencies to be zero due to the Born-Oppenheimer approximation being made in these calculations, in which we assume the nucleus to be stationary comparing to electrons.
Molecular Orbitals of BH3
Population analysis file: DOI:10042/88027
There are no significant differences between the real and LCAO MOs, although the real MOs tend to be more diffused, which indicates that the qualitative MO theory is quite accurate in predicting the general shapes of the MOs of small molecules.
NH3:B3LYP/6-31G(d,p) level
Optimisation log file here
| summary data | convergence | Jmol | |||
|---|---|---|---|---|---|

|
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000012 0.001800 YES RMS Displacement 0.000008 0.001200 YES |
|
Frequency file: here
| summary data | low modes |
|---|---|

|
Low frequencies --- -0.0130 -0.0028 -0.0003 7.0722 8.1014 8.1017 Low frequencies --- 1089.3849 1693.9369 1693.9369 |
Vibrational spectrum for NH3
| wavenumber (cm-1) | Intensity | IR active? | type |
| 1089 | 145 | yes | bend |
| 1694 | 14 | slight | bend |
| 1694 | 14 | slight | bend |
| 3461 | 1 | no | stretch |
| 3590 | 0 | no | stretch |
| 3590 | 0 | no | stretch |
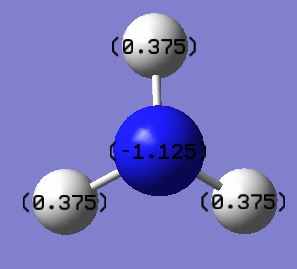
NBO analysis of NH3
Population analysis log file here
charge range -1.125 to 1.125
Day4
Association Energy of NH3BH3
NH3BH3:B3LYP/6-31G(d,p) level
Optimisation log file here
| summary data | convergence | Jmol | |||
|---|---|---|---|---|---|

|
Item Value Threshold Converged? Maximum Force 0.000002 0.000015 YES RMS Force 0.000001 0.000010 YES Maximum Displacement 0.000016 0.000060 YES RMS Displacement 0.000007 0.000040 YES |
|
Frequency file: here
| summary data | low modes |
|---|---|
|
Low frequencies --- -5.5307 -0.2567 -0.0370 -0.0002 1.4617 1.5147 Low frequencies --- 263.2923 632.9559 638.4634 |
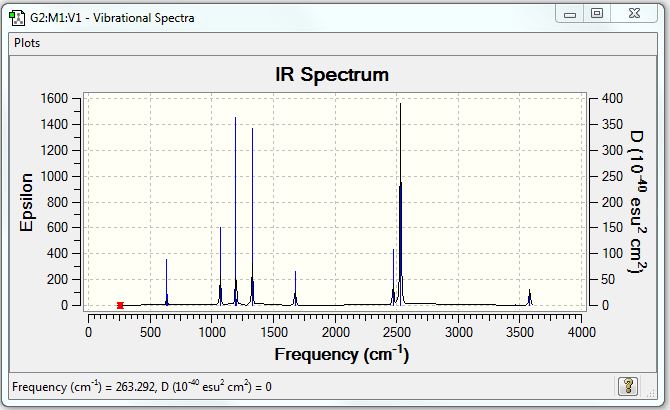
Vibrational spectrum for NH3BH3
| wavenumber (cm-1) | Intensity | IR active? | type |
| 263 | 0 | no | bend |
| 633 | 14 | slight | strecth |
| 638 | 4 | very slight | bend |
| 638 | 4 | very slight | bend |
| 1069 | 41 | yes | bend |
| 1069 | 41 | yes | bend |
| 1196 | 108 | yes | bend |
| 1204 | 3 | very slight | bend |
| 1204 | 3 | very slight | bend |
| 1329 | 114 | yes | bend |
| 1676 | 28 | slight | bend |
| 1676 | 28 | slight | bend |
| 2472 | 67 | yes | stretch |
| 2532 | 231 | yes | stretch |
| 2532 | 231 | yes | stretch |
| 3464 | 3 | very slight | stretch |
| 3581 | 28 | slight | stretch |
| 3581 | 28 | slight | stretch |
Association energy calculation
Note: The BH3:B3LYP/6-31G(d,p) optimisation reported earlier did not use the additional keywords 'scf=conver=9'. However, this keyword is used for the NH3 optimisation, therefore another optimisation of BH3 has been carried out and this additional keyword will be used for every calculation carried out later to improve accuracy.
Optimisation log file here
| summary data | convergence | Jmol | |||
|---|---|---|---|---|---|

|
Item Value Threshold Converged? Maximum Force 0.000004 0.000015 YES RMS Force 0.000003 0.000010 YES Maximum Displacement 0.000017 0.000060 YES RMS Displacement 0.000011 0.000040 YES |
|
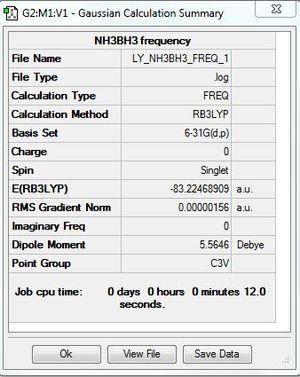
E(NH3)=-56.55776863 A.U.
E(BH3)=-26.61532364 A.U.
E(NH3BH3)=-83.22468909 A.U.
ΔE= -0.05159682 A.U. =-135 KJ/mol
The disassociation energy of NH3BH3 is greater than most of the hydrogen bonds and intermolecular attractions, however, it is still considerably smaller than most of the single covalent bond. The B-N bond enthalpy is smaller than half of the C-C bond enthalpy, but nearly the same as a Sn-Sn bond (150KJ/mol). Therefore, I would consider the B-N bond in NH3BH3 as a weak/medium bond.
Ionic Liquid Mini Project: Part 1
[N(CH3)4]+:B3LYP/6-31G(d,p) level
Optimisation and frequency analysis
Optimisation file: DOI:10042/96214
| summary data | convergence | Jmol | |||
|---|---|---|---|---|---|

|
Item Value Threshold Converged? Maximum Force 0.000008 0.000015 YES RMS Force 0.000002 0.000010 YES Maximum Displacement 0.000024 0.000060 YES RMS Displacement 0.000011 0.000040 YES |
|
Frequency file: DOI:10042/96226
| summary data | low modes |
|---|---|

|
Low frequencies --- 0.0006 0.0007 0.0007 21.3734 21.3734 21.3734 Low frequencies --- 188.5369 292.6576 292.6576 |
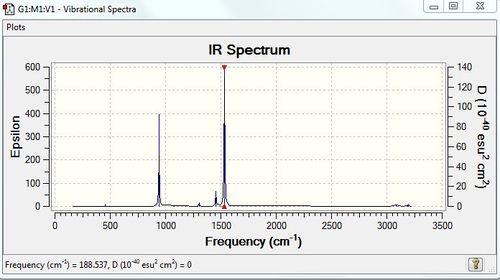
Vibrational spectrum for [N(CH3)4]+
| wavenumber (cm-1) | Intensity | IR active? | type |
| 941 | 22 | yes | bend |
| 941 | 22 | yes | bend |
| 941 | 22 | yes | bend |
| 1456 | 5 | very slight | bend |
| 1456 | 5 | very slight | bend |
| 1456 | 5 | very slight | bend |
| 1532 | 53 | yes | bend |
| 1532 | 53 | yes | bend |
| 1532 | 53 | yes | bend |
NBO analysis for [N(CH3)4]+
Population log file here
charge range -2 to 2
[P(CH3)4]+:B3LYP/6-31G(d,p) level
Optimisation and frequency analysis
Optimisation file: DOI:10042/96443
| summary data | convergence | Jmol | |||
|---|---|---|---|---|---|

|
Item Value Threshold Converged? Maximum Force 0.000002 0.000015 YES RMS Force 0.000001 0.000010 YES Maximum Displacement 0.000009 0.000060 YES RMS Displacement 0.000003 0.000040 YES |
|
Frequency file: DOI:10042/96454
| summary data | low modes |
|---|---|

|
Low frequencies --- 0.0006 0.0026 0.0035 24.6912 24.6912 24.6913 Low frequencies --- 160.0406 194.7811 194.7811 |
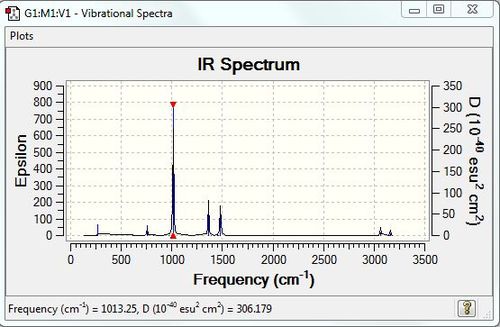
Vibrational spectrum for [P(CH3)4]+
Note: Only frequencies with intensities greater than 1 are analysed here and after.
| wavenumber (cm-1) | Intensity | IR active? | type |
| 271 | 2 | very slight | bend |
| 271 | 2 | very slight | bend |
| 271 | 2 | very slight | bend |
| 756 | 4 | very slight | bend |
| 756 | 4 | very slight | bend |
| 756 | 4 | very slight | bend |
| 1013 | 78 | yes | bend |
| 1013 | 78 | yes | bend |
| 1013 | 78 | yes | bend |
| 1362 | 21 | slight | bend |
| 1362 | 21 | slight | bend |
| 1362 | 21 | slight | bend |
| 1481 | 26 | slight | bend |
| 1481 | 26 | slight | bend |
| 1481 | 26 | slight | bend |
| 3064 | 5 | very slight | stretch |
| 3064 | 5 | very slight | stretch |
| 3064 | 5 | very slight | stretch |
| 3159 | 4 | very slight | stretch |
| 3159 | 4 | very slight | stretch |
| 3159 | 4 | very slight | stretch |
NBO analysis for [P(CH3)4]+
Population log file here
charge range -2 to 2
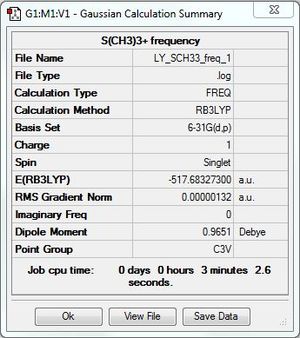
[S(CH3)3]+:B3LYP/6-31G(d,p) level
Optimisation and frequency analysis
Optimisation log file: DOI:10042/101299
| summary data | convergence | Jmol | |||
|---|---|---|---|---|---|

|
Item Value Threshold Converged? Maximum Force 0.000002 0.000015 YES RMS Force 0.000001 0.000010 YES Maximum Displacement 0.000023 0.000060 YES RMS Displacement 0.000011 0.000040 YES |
|
Frequency file: DOI:10042/101323
| summary data | low modes |
|---|---|
|
Low frequencies --- -8.0125 -7.8188 -7.8156 0.0022 0.0043 0.0093 Low frequencies --- 161.8459 199.5841 199.5842 |
Vibrational spectrum for [S(CH3)3]+
| wavenumber (cm-1) | Intensity | IR active? | type |
| 624 | 2 | very slight | stretch |
| 704 | 1 | very slight | stretch |
| 704 | 1 | very slight | stretch |
| 958 | 1 | very slight | bend |
| 958 | 1 | very slight | bend |
| 1071 | 11 | slight | bend |
| 1071 | 11 | slight | bend |
| 1076 | 12 | slight | bend |
| 1408 | 2 | very slight | bend |
| 1464 | 10 | slight | bend |
| 1464 | 10 | slight | bend |
| 1473 | 25 | yes | bend |
| 1473 | 25 | yes | bend |
| 1485 | 42 | yes | bend |
| 3075 | 3 | very slight | stretch |
| 3075 | 3 | very slight | stretch |
| 3185 | 8 | slight | stretch |
| 3185 | 8 | slight | stretch |
| 3187 | 3 | very slight | stretch |
| 3187 | 2 | very slight | stretch |
| 3187 | 2 | very slight | stretch |
NBO analysis for [S(CH3)3]+
Population log file here
charge range -2 to 2
[X(CH3)y]+ Geometry Comparison Section
| [N(CH3)4]+ | [P(CH3)4]+ | [S(CH3)3]+ | |
|---|---|---|---|
| r(X-C) Å | 1.51 | 1.82 (1.816 in 3 d.c.) | 1.82 (1.822 in 3 d.c.) |
| r(C-H) Å | 1.09 | 1.09 | 1.09 |
| θ(C-X-C) degrees(º) | 109.5 | 109.5 | 102.7 |
| θ(H-C-H) degrees(º) | 110.0 | 109.0 | 109.4 or 111.1* |
| θ(H-C-X) degrees(º) | 108.9 | 109.9 | 110.6 or 107.3** |
* & ** The two values in these two cells are due to the two types of equivalent H atoms in [S(CH3)3]+. Of the total 9 H in [S(CH3)3]+, 6 equivalent ones point upwards (i.e. closer to central S atom) and the other 3 equivalent ones point away from the central S atom.
The Pauling scale electronegativities of carbon, nitrogen, phosphorus and sulphur atoms are 2.55, 3.04, 2.19, 2.58, respectively.[3] The atomic radii of carbon, nitrogen, phosphorus and sulphur are 76.7 70.2, 108.8, 105.2 pm respectively.[4]
Both [N(CH3)4]+ and [P(CH3)4]+ adopt tetrahedral geometry and the C atoms in these two cations adopt slightly distorted tetrahedral geometry. This is because both of these cations have four totally symmetrical -CH3 groups. An overall tetrahedral geometry can reduce the total energy to the minimum and this can be confirmed by both the optimisation summary and the C-X-C bond angle (109.5º, which is the same as H-C-H bond angle in methane). The bond length of N-C bond is significantly shorter than the P-C bond which indicates that the N-C bond is much stronger than the P-C bond. This is because that both N and C are second row elements and they are in fact next to each other, whereas P is a third row element, therefore there is a smaller energy mismatch between the atomic orbitals of N and C than that of P and C, resulting in larger overlap between orbital of N and C, and ultimately stronger bonding MO. The size of P is also larger than N which furthermore sterically increases the P-C bond length. The larger central atom in [P(CH3)4]+ also sterically pushed the three C-H bonds towards each other a little bit as well, which increases the H-C-X bond angle and decreases the H-C-H bond angle.
[S(CH3)3]+ adopts a trigonal pyramidal shape and the C atoms it adopts slightly distorted tetrahedral geometry. The lone pair of electron on the S atom pushed the the three -CH3 closer to each other than in a tetrahedral shape which reduces the C-X-C bond angle to 102.7º. Overall, the structure of [S(CH3)3]+ is more like an analogue to ammonia whereas the structures of [N(CH3)4]+ and [P(CH3)4]+ are analogues to methane. The S-C bond length is slightly longer than the P-C bond length probably due to less steric hindrance and repulsion from the lone pair of [S(CH3)3]+, which allows the S-C bond to elongate a bit. The values of H-C-H and H-C-X bond angles can be explained by the repulsion between the lone pair on S and the C-H bond. For each -CH3 in the [S(CH3)3]+ cation, 2 H atoms point upwards from the base of the trigonal pyramid ( close to S atom) and 1 H atom point downwards from the trigonal pyramid (away from S atom). The two upwards C-H bonds are repelled by the lone pair on S more strongly and hence resulting in the large H-C-X (110.6º) bond angle and small H-C-H bond angle (109.4º) between the two of them.
The C-H bond lengths in all the cations are nearly identical, which suggests that the central heteroatom has a negligible effect on the strength of C-H bond. This is expected as the only influence that the central heteroatom can exert on the C-H bond is through induction rather than resonance and induction effect is generally small.
MOs of [N(CH3)4]+
select 5 and comment
Are AO interactions strong or weak?
Are AO interactions bonding or antibonding?
Are there through space interactions?
How many and what kinds of nodes are there?
How delocalised is the MO?
The orbitals listed below is in an order from strongly bonding to strongly anti-bonding
MO6 : strong bonding
This MO is the first non-core MO of [N(CH3)4]+. The shape of this this MO is like a 'concave balloon' and this may be caused by the electronegative N in the center which can suck electron density towards the center. Overall, this MO is quite delocalised but may not be as diffused as its analogous MO in isobutane.
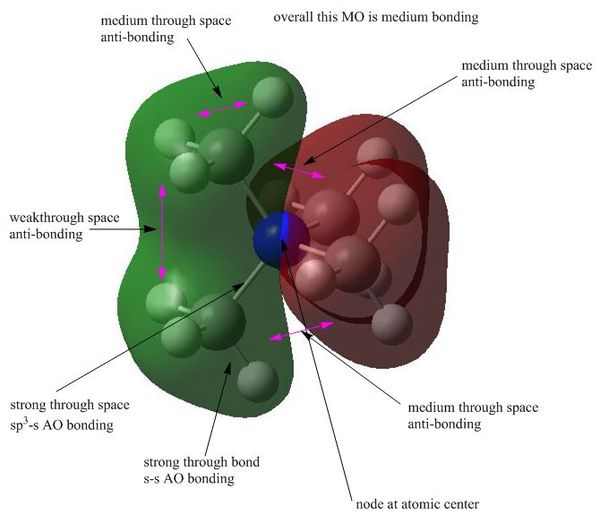
MO7 : medium bonding
This MO is triply degenerate and hence the p orbitals of central N atom has probably participated in the formation of this MO. The node at N atom is not as important as a nodal plane. The two lobes of orbitals are actually perpendicular to each other, which lowers the anti-boning interaction in the middle of the cation. Therefore, this bond is overall medium boding. The size of this MO is also slightly bigger and more delocalised than MO6, which is caused by the anti-bonding interactions in the center and p orbitals involved in forming it as p orbitals are naturally larger than s orbitals.
MO13 : weak bonding
This MO is triply degenerate and contains 5 nodes at atomic centers. This MO is separated into two bent lobes and within each lobe considerable amount of through space bonding interaction is seen. All the nodes are at atomic centers which are not as significant as nodal planes. This MO is more delocalised than MO6 but probably more localised than all the MOs shown below as central N atom can still constrain a significant amount of electron density of this MO.
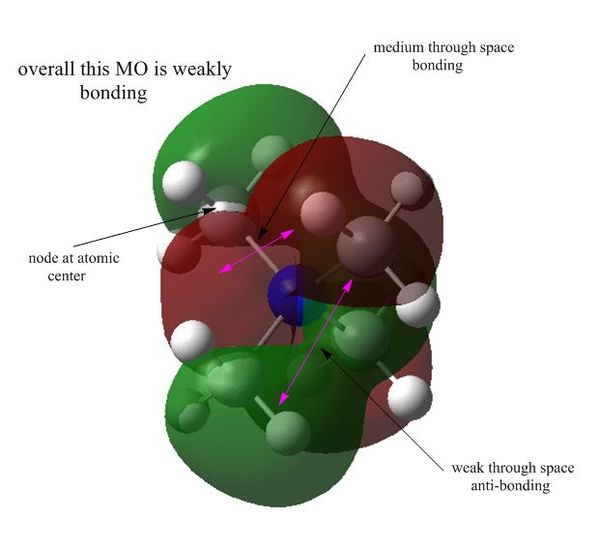
MO15 : weak anti-bonding
This MO is doubly degenerate and contains two nodal planes and four nodes at atomic centers. Although there are through space bonding interaction within each lobe, the strong through space anti-bonding interactions make this MO weakly anti-bonding overall. Each lobe of this orbital is more delocalised as AOs from the central N atom are not involved in forming this MO.
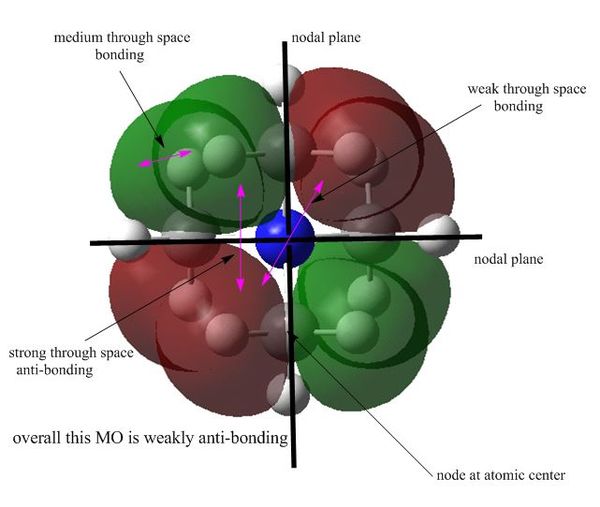
MO16 : strong anti-bonding
This MO is triply degenerate and probably caused by the p orbitals of C atoms. This MO contains 4 nodes at atomic centers as well as 3 nodal planes. Although weak through space bonding interactions between diagonal lobes, overall it is a strong anti-bonding MO. The 8 lobes are all leaning outwards which is caused by the repulsion between lobes of different phase. This MO is also quite localised comparing to other MOs as all the lobes only cover two to three atoms.
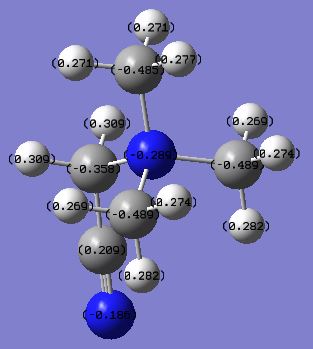
[X(CH3)y]+ Charge Distribution Comparison Section
| [N(CH3)4]+ | [P(CH3)4]+ | [S(CH3)3]+ | |
|---|---|---|---|
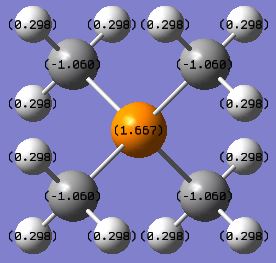
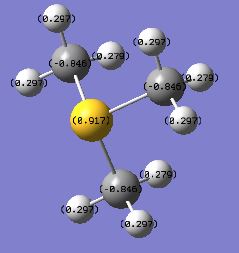
| charge on X | -0.295 | 1.667 | 0.917 |
| charge on C | -0.483 | -1.060 | -0.846 |
| charge on H | 0.269 | 0.298 | 0.297 or 0.279* |
* The hydrogen atoms in [S(CH3)3]+ have two different values of positive charges due to the existence of two types of equivalent H in this cation. The 6 H atoms pointing upwards (i.e. closer to the central S atom) have more positive charges (0.297), whereas the 3 H atoms pointing away from the central S atom have less positive charges(0.279).
Compare and contrast the charge distribution across this trio of cations
C atoms in all of these structures are negatively charged with a trend that the more electronegative the central atom is the less negatively charged the C atoms are. This is because the more electronegative the central atom is, the stronger the attraction exerted on electron density in the C-X bond from the central atom, which reduces the electron density on the hydrogen atom. However, even when the central atom is the most electronegative N, C is still negatively charged, which is probably due to the 3 electropositive H atoms attached to the C atom.
H atoms in all of these cations are positively charged with similar values. However the more electronegative the central atom is, the less positively charged the H atom is. This is probably due to the size of the central atoms. The least electronegative central atom, P, is the largest one among the three cations, which means that it can spatially accommodate more electron density. Although the electron density is more diffused on P atom, it can suck more electron density from the terminal H atom. S atom is only slightly smaller than the P atom and hence the positive charges on the hydrogens of the S centred cation are just slightly smaller than the hydrogens of the P centred cation, especially for the 6 hydrogens that are closer to the central S atom. Since N is in the second row of the periodic table and hence a significantly smaller atom, the hydrogens of the N centred cation are much less positively charged.
The charge on the central atom is just a measure of its electronegativity. The most electronegative N atom has a negative charge of -0.295, whereas the least electronegative P has a positive charge of 1.667.
[X(CH3)y]+ Relative Contribution form C and X to the C-X Bond Comparison Section
| [N(CH3)4]+ | [P(CH3)4]+ | [S(CH3)3]+ | |
|---|---|---|---|
| Contribution from X | 66% | 40% | 51% |
| Hybridisation of X orbital | 25% s + 75% p | 25% s + 74% p + 1% d | 17% s + 82% p + 1% d |
| Contribution from C | 34% | 60% | 49% |
| Hybridisation of C orbital | 21% s + 79% p | 25% s + 75% p | 20% s + 80% p |
compare and contrast the relative contribution of the C and heteroatom to the C-X bond. How do your results relate to the charge distribution just studied?
The orbitals form C, N, P, S that contribute to the C-X bonds are all roughly sp3 hybridised. However, the orbitals of P and S have a tiny bit of d orbital character because they are third row atoms, which means that 3d orbital is available to them.
From the table above, it can be seen as a general trend that the more electronegative atom between the C and X pair contributes more to the C-X bond. For example, N is more electronegative than C (3.04 vs. 2.55), and hence N contributes more to the C-N bond than C( 66% vs. 34%). This is because the more electronegative atom can attract more electron density than the less electronegative one, i.e. the electron density is more polarised towards the more electronegative atom, and therefore the more electronegative atom contributes more to the C-X bond. From a MO theory's point of view, the orbital that is used to construct the C-X bond of the more electronegative atom is lower in energy than the less electronegative one, which leads to the resulting bonding MO being closer in energy and similar in shape to the orbital of the more electronegative atom. Hence, we get the same conclusion that the more electronegative atom contributes more to the C-X bond.
Validity of the traditional [N(R4]+ description
What does the "formal" positive charge on the N represent in the traditional picture?On what atoms is the positive charge actually located for this cation?
The concept of formal charge is based on the traditional Lewis structure, in which electron pair is considered to be equally shared between any two bonding atoms regardless of their electronegativities. The definition of formal charge is therefore, the difference between the positive nuclear charge and the total number of electrons of an atom in a given molecule. [5]
Based on this definition, the N atom in [N(R4]+ has a nuclear charge of 7 and total number of electrons of 6, of which 2 belong to the non-bonding inner shell (1s) and 4 are shared with the atoms in the R groups. Therefore, it has a formal charge of +1.
However, because the concept of Lewis structure does not take electronegativities into account, the assignment of +1 charge to the N atom in the case of [N(CH3)4]+ is not correct. The +1 positive charge is actually spread out over all of the H atoms in this cation.
Ionic Liquid Mini Project: Part 2
[N(CH3)3(CH2OH)]+:B3LYP/6-31G(d,p) level
Optimisation and frequency analysis
Optimisation file: DOI:10042/104254
| summary data | convergence | Jmol | |||
|---|---|---|---|---|---|

|
Item Value Threshold Converged? Maximum Force 0.000002 0.000015 YES RMS Force 0.000000 0.000010 YES Maximum Displacement 0.000041 0.000060 YES RMS Displacement 0.000014 0.000040 YES |
|
Frequency file: DOI:10042/104258
| summary data | low modes |
|---|---|

|
Low frequencies --- -8.4389 -5.0421 -1.1038 -0.0009 -0.0008 -0.0008 Low frequencies --- 131.1013 213.4668 255.7136 |
Interesting aspect of the structure of [N(CH3)3(CH2OH)]+
During the optimisation of [N(CH3)3(CH2OH)]+, another structure came out initially, with O-H bond being antiperiplanar to the C-N bond, of which the C is the OH directly attached to. Despite the fact that this structure has a CV symmetry, which is higher in symmetry than the structure presented above (C1, further frequency analysis showed that this structure is not a minimum as it has a imaginary frequency.
The reason why [N(CH3)3(CH2OH)] prefers a low symmetry structure may be explained by the attraction between O atom and 2H atoms on adjacent carbon atoms (The two H atoms that are pointing in the same direction as the O atom on adjacent C atoms). The maximum attraction between non-bonded H and O typically occurs at 2.62 Å, estimated in the basis of their Van Der Waals's radii.[6]
In the CV symmetry structure, the distances between O and two H are both 2.41 Å, whereas these distances for the C1 symmetry structure are 2.55 and 2.46 Å. Therefore, clearly that the attraction between non-bonded O and H in the low symmetry structure is stronger and hence it is lower in energy. In fact, if we examine the imaginary frequency of the high symmetry structure, it corresponds to a bending motion that moves the hydroxyl H to the position that it sits in the low symmetry structure.
Vibrational spectrum for [N(CH3)3(CH2OH)]+
| wavenumber (cm-1) | Intensity | IR active? | type |
| 131 | 5 | very slight | bend |
| 213 | 3 | very slight | bend |
| 256 | 29 | yes | bend |
| 267 | 1 | very slight | bend |
| 342 | 51 | yes | bend |
| 355 | 4 | very slight | bend |
| 393 | 28 | yes | bend |
| 434 | 4 | very slight | bend |
| 449 | 4 | very slight | bend |
| 552 | 2 | very slight | bend |
| 736 | 22 | yes | stretch |
| 839 | 96 | yes | bend |
| 931 | 22 | yes | bend |
| 982 | 12 | slight | stretch |
| 1033 | 20 | yes | bend |
| 1122 | 38 | yes | bend |
| 1133 | 7 | slight | bend |
| 1184 | 91 | yes | stretch |
| 1219 | 8 | slight | bend |
| 1276 | 6 | very slight | bend |
| 1289 | 2 | very slight | stretch |
| 1330 | 19 | yes | bend |
| 1397 | 17 | yes | bend |
| 1433 | 3 | very slight | bend |
| 1445 | 7 | slight | bend |
| 1452 | 9 | slight | bend |
| 1496 | 5 | very slight | bend |
| 1501 | 3 | very slight | bend |
| 1504 | 1 | very slight | bend |
| 1514 | 26 | yes | bend |
| 1521 | 33 | yes | bend |
| 1530 | 17 | yes | bend |
| 1540 | 51 | yes | bend |
| 3074 | 9 | slight | stretch |
| 3085 | 2 | very slight | stretch |
| 3088 | 2 | very slight | stretch |
| 3095 | 1 | very slight | stretch |
| 3147 | 4 | very slight | stretch |
| 3184 | 1 | very slight | stretch |
| 3189 | 1 | very slight | stretch |
| 3825 | 105 | yes | stretch |
NBO analysis for [N(CH3)3(CH2OH)]+
Population log file here
charge range -2 to 2
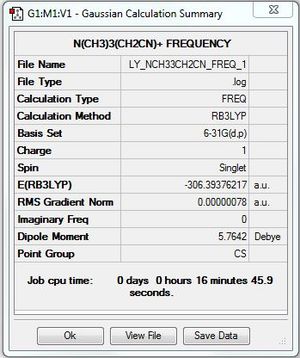
[N(CH3)3(CH2CN)]+:B3LYP/6-31G(d,p) level
Optimisation and frequency analysis
Optimisation file: DOI:10042/101786
| summary data | convergence | Jmol | |||
|---|---|---|---|---|---|

|
Item Value Threshold Converged? Maximum Force 0.000001 0.000015 YES RMS Force 0.000000 0.000010 YES Maximum Displacement 0.000016 0.000060 YES RMS Displacement 0.000005 0.000040 YES |
|
Frequency file: DOI:10042/101797
| summary data | low modes |
|---|---|
|
Low frequencies --- -3.6804 0.0006 0.0008 0.0009 2.7400 3.8945 Low frequencies --- 91.8704 153.9681 212.1245 |
Vibrational spectrum for [N(CH3)3(CH2CN)]+
| wavenumber (cm-1) | Intensity | IR active? | type |
| 92 | 6 | slight | bend |
| 154 | 9 | slight | bend |
| 328 | 1 | very slight | bend |
| 436 | 1 | very slight | bend |
| 571 | 2 | very slight | bend |
| 895 | 28 | yes | bend |
| 911 | 20 | yes | stretch |
| 963 | 14 | slight | stretch |
| 990 | 20 | yes | stretch |
| 1008 | 2 | very slight | bend |
| 1140 | 1 | very slight | bend |
| 1259 | 1 | very slight | bend |
| 1333 | 3 | very slight | bend |
| 1395 | 8 | slight | bend |
| 1454 | 8 | slight | bend |
| 1455 | 8 | slight | bend |
| 1475 | 3 | very slight | bend |
| 1495 | 3 | very slight | bend |
| 1503 | 3 | very slight | bend |
| 1519 | 34 | yes | bend |
| 1521 | 47 | yes | bend |
| 1532 | 61 | yes | bend |
| 2385 | 8 | slight | stretch |
| 3087 | 1 | very slight | stretch |
| 3144 | 2 | stretch | bend |
Note: The peak at 92 cm-1 shows an unexpected large peak on the spectrum generated by Gaussview. The calculation has been re-run several times and the error persisted. I have had a discussion about this error with Dr. Hunt, but we still couldn't figure out where the error comes from.
NBO analysis for [N(CH3)3(CH2CN)]+
Population log file here
charge range -2 to 2
Charge distribution comparison between [N(CH3)3(CH2OH)]+ and [N(CH3)3(CH2CN)]+
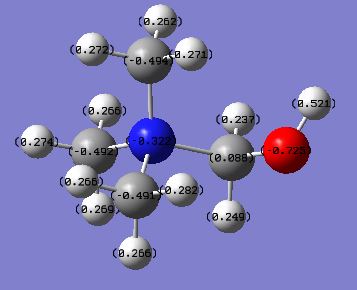
| [N(CH3)4]+ | [N(CH3)3(CH2OH)]+ | [N(CH3)3(CH2CN)]+ | |
|---|---|---|---|
| charge on N | -0.295 | -0.322 | -0.289(central), -0.186(CN) |
| charge on C | -0.483 | -0.494 ~ -0.491(CH3), 0.088(CH2OH) | -0.385 ~ -0.489(CH3), 0.209((CN) |
| charge on O | N/A | -0.725 | N/A |
| charge on H | 0.269 | 0.266 ~ 0.282(CH3), 0.237 ~ 0.249(CH2OH), 0.521(CH2OH) | 0.269 ~ 0.282(CH3), 0.309(CH2CN) |
OH is an electron donating group and CN is an electron withdrawing group, what effect have these groups had on the charge distribution?
From the charge distribution data, it is easy to discover that OH has pushed electron density across the ammonium cation, which increases the negative charge on the central N atom and most of the carbon atom apart from the one which it is directly attached to. The positive charge on the H atoms in [N(CH3)3(CH2OH)] has also been decreased apart from the hydroxyl H. Generally, the closer the H atom to the OH group, the less positive the H is.
On the other hand, the CN group has significantly withdrawn electron density from both the central N atom and most of the carbon atom apart from the one which it is directly attached to. As a result, the negative charge on both the central N and C atoms has decreased comparing to the reference [N(CH3)4]+. The positive charges on the H atoms in [N(CH3)3(CH2CN)]+ have also increased and the amount of increases have a negative correlation with the distance between the H and the CN.
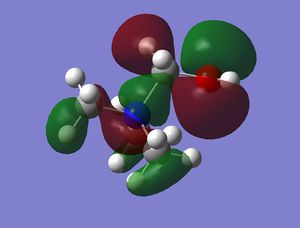
HOMO and LUMO comparison
| [N(CH3)4]+ | [N(CH3)3(CH2OH)]+ | [N(CH3)3(CH2CN)]+ | |
|---|---|---|---|
| HOMO |  |
 |
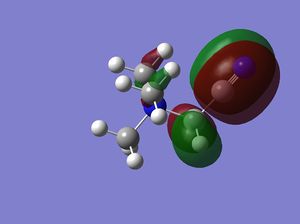
|
| LUMO | 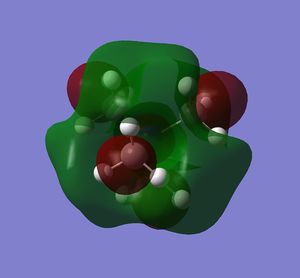 |
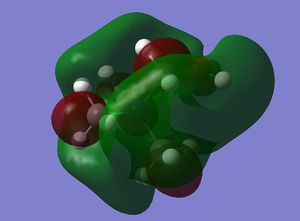 |
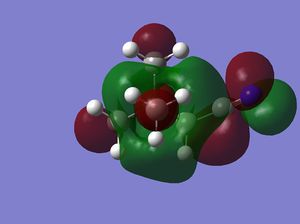
|
Note: The HOMO of [N(CH3)4]+ is triply degenerate.
| [N(CH3)4]+ | [N(CH3)3(CH2OH)]+ | [N(CH3)3(CH2CN)]+ | |
|---|---|---|---|
| HOMO (Hartree) | -0.57934 | -0.48763 | -0.50047 |
| LUMO (Hartree) | -0.13303 | -0.12459 | -0.18183 |
| HOMO-LUMO gap (KJ•mol-1) | 1172 | 953 | 837 |
how has the shape of the orbitals changed?
has the energy of these orbitals moved?
has the HOMO-LUMO gap changed in size?
what chemical impact could these changes have?
The HOMO of [N(CH3)4]+ is triply degenerate due to the tetrahedral geometry of the cation. By introducing the OH or CN, the symmetries of the HOMOs are broken and HOMO orbitals are more localised and contracted as well. The latter is due to the existence of the electronegative O and N which can suck in electron densities. The HOMO of [N(CH3)3(CH2CN)]+ is actually more localised than [N(CH3)3(CH2OH)]+, probably due to the electron withdrawing nature of CN. This effect is more obvious on the LUMO orbitals. The LUMO of [N(CH3)3(CH2CN)]+ is significantly more contracted than that of the [N(CH3)3(CH2OH)]+.
The energies of HOMO orbitals have increased for both [N(CH3)3(CH2OH)]+ and [N(CH3)3(CH2CN)]+ due to the occurrence of nodal planes in these two cations, which indicates an increased amount of anti-bonding interactions.
On the other hand, the energies of LUMO orbitals have increased for both [N(CH3)3(CH2OH)]+ but decreased for [N(CH3)3(CH2CN)]+, which means that [N(CH3)3(CH2CN)]+ is more susceptible to nucleophilic attack than [N(CH3)3(CH2OH)]+. This is consistent with our chemical intuitions as CN is general a better leaving group than OH.
The LUMO-HOMO gap has also decreased for both [N(CH3)3(CH2OH)]+ and [N(CH3)3(CH2CN)]+, which means that both of them are more liable to photo and thermal exaction than [N(CH3)4]+.
Reference
- ↑ IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). XML on-line corrected version: http://goldbook.iupac.org (2006-) created by M. Nic, J. Jirat, B. Kosata; updates compiled by A. Jenkins. ISBN 0-9678550-9-8. doi:10.1351/goldbook. (Accessed on 20/11/2014)
- ↑ P. Atkins, T. Overton, J. Rourke, M. Weller and F. Armstrong, Shriver & Atkins's Inorganic Chemistry, 5th edn.
- ↑ A.L. Allred, J. Inorg. Nucl. Chem., 17, 215 (1961).
- ↑ F.H.. Allen, O. Kennard, D.G. Watson, L. Brammer, A.G. Orpen and R. Taylor, J. Chem. Soc. Perkin II, S1 (1987).
- ↑ IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). XML on-line corrected version: http://goldbook.iupac.org (2006-) created by M. Nic, J. Jirat, B. Kosata; updates compiled by A. Jenkins. ISBN 0-9678550-9-8. doi:10.1351/goldbook.L03513. (Accessed on 19/11/2014.)
- ↑ M. Mantina, A.C. Chamberlin, R. Valero, C.J. Cramer and D.J. Truhlar, J. Phys. Chem. A , 113(19), 2009.