Rep:Mod:InorgWeek1 js6511 2013-14
3rd Year Inorganic Computational Chemistry Lab - Week 1
James Spreadborough 00690768
Initial Optimisations
BH3 Optimisation (3-21G)
Gaussian 09W was used to calculate the optimised molecule of BH3 with 3-21G basis set:
This is the link to the .log result file:
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis set | 3-21G |
| Charge | 0 |
| Spin | Singlet |
| Final Energy | -26.4031263994 a.u. |
| Gradient | 0.000088513 a.u. |
| Dipole Moment | 0.0003 D |
| Point Group | Cs |
| Calculation Time | 14.0 seconds |
The Item table confirmed that the forces converged at the end of the calculation:
Item Value Threshold Converged? Maximum Force 0.000220 0.000450 YES RMS Force 0.000106 0.000300 YES Maximum Displacement 0.000709 0.001800 YES RMS Displacement 0.000447 0.001200 YES Predicted change in Energy=-1.672479D-07 Optimization completed. -- Stationary point found.
The bond length values from this optimisation was quite close to the literature value of 1.1900 Å[1], suggesting that this optimisation is still quite accurate in determining optimum molecular structures. The angles were very to the trigonal planar value of 120° as well.
| Name | Value | Value |
|---|---|---|
| R1 | R(1,2) | 1.1947 |
| R2 | R(1,3) | 1.1944 |
| R3 | R(1,4) | 1.1948 |
| A1 | A(2,1,3) | 119.9983 |
| A2 | A(2,1,4) | 120.0157 |
| A3 | A(3,1,4) | 119.986 |
| D | D(2,1,4,3) | 180 |
The energies of each optimisation step decrease as the optimisation refines the molecule to find the lowest energy equilibrium structure. The gradient values also decrease as the calculation tries to find the minimum point where the equilibrium structure lies, with the final step having the smallest energy and gradient values.

The structures of the optimisation steps can be visualised with models, and in this case the first three steps were not considered by Gaussian to have formed bonds between their atoms because the distances between the atoms were too large. The differences in energy and gradient however, especially for the third step, are still close to those for the others and may well still form strong enough attractions to be called a chemical bond.
| Structure at 1st step | Structure at 7th step |
|---|---|
 |

|
BH3 Optimisation (6-31G (d,p))
The optimised molecule of BH3 with a 6-31G (d,p) basis set was then calculated:
This is the link to the .log result file:
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis set | 6-31G |
| Charge | 0 |
| Spin | Singlet |
| Final Energy | -26.61532358 a.u. |
| Gradient | 0.00008206 a.u. |
| Dipole Moment | 0.0003 D |
| Point Group | Cs |
| Calculation Time | 20.0 seconds |
The Item table confirmed that the forces converged at the end of the calculation:
Item Value Threshold Converged? Maximum Force 0.000204 0.000450 YES RMS Force 0.000099 0.000300 YES Maximum Displacement 0.000659 0.001800 YES RMS Displacement 0.000418 0.001200 YES Predicted change in Energy=-1.452164D-07 Optimization completed. -- Stationary point found.
The bond lengths determined by this method were closer to the literature value[1] than with the 3-21G basis set, implying that this better for the optimisation calculations. The bond angles were very close to but not exactly 120°, similar to the first basis set.
| Name | Value | Value |
|---|---|---|
| R1 | R(1,2) | 1.1926 |
| R2 | R(1,3) | 1.1924 |
| R3 | R(1,4) | 1.1928 |
| A1 | A(2,1,3) | 119.9988 |
| A2 | A(2,1,4) | 120.0146 |
| A3 | A(3,1,4) | 119.9866 |
| D | D(2,1,4,3) | 180 |
GaBr3 Optimisation
The optimised molecule of GaBr3 with a LANL2DZ basis set and D3h restricted symmetry was then calculated:
This is the link to the .log result file:
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis set | LANL2DZ |
| Charge | 0 |
| Spin | Singlet |
| Final Energy | -41.70082783 a.u. |
| Gradient | 0.00000016 a.u. |
| Dipole Moment | 0.0000 D |
| Point Group | D3H |
| Calculation Time | 16.0 seconds |
The Item table confirmed that the forces converged at the end of the calculation:
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000003 0.001800 YES RMS Displacement 0.000002 0.001200 YES Predicted change in Energy=-1.282683D-12 Optimization completed. -- Stationary point found.
The value of 2.3502 Å was reasonably agreeable with the literature value of 2.249 Å[2], although not nearly as accurate as either basis set had been for the smaller BH3 molecule. The bond angles were, however, exactly 120°, perfect for a trigonal planar molecule. This was due to the restricted symmetry placed on the molecule at the beginning of the calculation, forcing it into a D3h conformation.
| Name | Value | Value |
|---|---|---|
| R1 | R(1,2) | 2.3502 |
| R2 | R(1,3) | 2.3502 |
| R3 | R(1,4) | 2.3502 |
| A1 | A(2,1,3) | 120.0 |
| A2 | A(2,1,4) | 120.0 |
| A3 | A(3,1,4) | 120.0 |
| D | D(2,1,4,3) | 180 |
BBr3 Optimisation
The optimised molecule of BBr3 was then calculated with a GEN basis set and "pseudo=read gfinput" keywords:
This is the link to the .log result file:
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis set | GEN |
| Charge | 0 |
| Spin | Singlet |
| Final Energy | -64.43645296 a.u. |
| Gradient | 0.00000382 a.u. |
| Dipole Moment | 0.0000 D |
| Point Group | D3H |
| Calculation Time | 21.4 seconds |
The Item table confirmed that the forces converged at the end of the calculation:
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000005 0.000300 YES Maximum Displacement 0.000036 0.001800 YES RMS Displacement 0.000023 0.001200 YES Predicted change in Energy=-4.027258D-10 Optimization completed. -- Stationary point found.
The bond length was calculated as 1.934 Å, and this agreed with the literature value of 1.893 Å[3] fairly well. The 120° bond angles are also accurate with the trigonal planar structure the molecule has.
| Name | Value | Value |
|---|---|---|
| R1 | R(1,2) | 1.934 |
| R2 | R(1,3) | 1.934 |
| R3 | R(1,4) | 1.934 |
| A1 | A(2,1,3) | 120.0 |
| A2 | A(2,1,4) | 120.0 |
| A3 | A(3,1,4) | 120.0 |
| D | D(2,1,4,3) | 180 |
BH3, GaBr3 and BBr3 Bond Lengths
| Molecule | Bond Length (Å) |
|---|---|
| BH3 | 1.1926 |
| GaBr3 | 2.3502 |
| BBr3 | 1.934 |
Changing the ligand from H to Br increases the bond length, almost by twice as much. This could be down to the larger size of bromine causing more repulsive forces, but the strongest factor here is probably the electronegativity difference. The B-H electronegativity difference is 0.16 (from the Pauling Scale), giving quite a non-polar bond which will be quite strong, and therefore short. The B-Br electronegativity difference is 0.92 however, a much bigger difference and much more polar, almost ionic, bond that lengthens and weakens the bond.
Changing the central atom from B to Ga also increases the bond length, but not by as much. The larger size of gallium causing more repulsions and a longer bond is again a factor here. Boron has the same ionic state as Ga, 3+, but has 5 electrons compared to 31 for gallium. This means that boron has a much higher charge density and has much better orbital overlap with bromine than gallium, forming a stronger and shorter bond. There is also a smaller electronegativity difference for B-Br (0.92) than for Ga-Br (1.15), giving B-Br a slightly more non-polar and shorter bond.
Frequency Analysis
BH3 Frequency Analysis
For BH3 the 6-31G (d,p) optimisation was first repeated, but with scf=conver=9 and int=ultrafine keywords, to gain a better optimisation that wouldn't lead to negative frequencies. This is the link to the .log file:
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Charge | 0 |
| Spin | Singlet |
| Final Energy | -26.61532360 a.u. |
| Gradient | 0.00000040 a.u. |
| Dipole Moment | 0.0000 D |
| Point Group | Cs |
| Calculation Time | 58.0 seconds |
The Item table confirmed that the forces converged at the end of the calculation:
Item Value Threshold Converged? Maximum Force 0.000001 0.000015 YES RMS Force 0.000000 0.000010 YES Maximum Displacement 0.000004 0.000060 YES RMS Displacement 0.000003 0.000040 YES Predicted change in Energy=-3.721869D-12 Optimization completed. -- Stationary point found.
| Name | Value | Value |
|---|---|---|
| R1 | R(1,2) | 1.1923 |
| R2 | R(1,3) | 1.1923 |
| R3 | R(1,4) | 1.1923 |
| A1 | A(2,1,3) | 120.0 |
| A2 | A(2,1,4) | 120.0002 |
| A3 | A(3,1,4) | 119.9997 |
| D | D(2,1,4,3) | 180 |
Gaussian 09W was then used to calculate the frequency of BH3 to find the vibrational modes and IR spectrum of the molecule, and this is the link to the .log result file:
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Charge | 0 |
| Spin | Singlet |
| Final Energy | -26.61532364 a.u. |
| Gradient | 0.00000016 a.u. |
| Dipole Moment | 0.0000 D |
| Point Group | D3h |
| Calculation Time | 38.0 seconds |
The Item table confirmed that the forces converged at the end of the calculation:
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000001 0.001800 YES RMS Displacement 0.000001 0.001200 YES Predicted change in Energy=-6.027773D-13 Optimization completed. -- Stationary point found.
The calculated vibrational frequencies were within the ±15cm-1 range:
Low frequencies --- -9.5530 -9.5386 -0.1141 0.0009 0.5250 1.6148 Low frequencies --- 1162.9891 1213.1488 1213.1490
The vibrational modes of the molecule were animated and are compared in the table below.
| No. | Form of the vibration | Frequency | Intensity | Symmetry (D3h Point Group) |
| 1 | All three H atoms wag up and down, with the B atom still | 1162.99 | 92.5667 | A2' |
| 2 | Asymmetric wagging in plane of molecule with two H atoms moving clockwise whilst the other moves anticlockwise | 1213.15 | 14.0556 | E' |
| 3 | Symmetric wagging in plane of molecule, with only two H atoms moving and the others still | 1213.15 | 14.0551 | E' |
| 4 | Symmetric stretching from all three H atoms | 2582.53 | 0.0000 | A1' |
| 5 | Anti-symmetric stretching from just two of the H atoms | 2715.66 | 126.3338 | E' |
| 6 | Asymmetric stretching with two H atoms stretching at a different time to the third one | 2715.66 | 126.3278 | E' |
The IR spectrum was also attained from the frequency calculations:

There are six vibrations in the molecule, but only three peaks in the IR spectrum. The first vibration clearly has its own peak, and is quite intense, but the second and third vibrations share the same frequency and very similar intensity, so are only seen as one peak. The fourth vibration has no intensity because it is a completely symmetrical vibration and the stretches cancel out, leaving no peak. The fifth and sixth vibrations are also at the same frequency and intensity, giving only three peaks in total.
GaBr3 Frequency Analysis
Gaussian 09W was then used to calculate the frequency of GaBr3, and this is the link to the .log file:
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis set | LANL2DZ |
| Charge | 0 |
| Spin | Singlet |
| Final Energy | -41.70082783 a.u. |
| Gradient | 0.00000011 a.u. |
| Dipole Moment | 0.0000 D |
| Point Group | D3h |
| Calculation Time | 8.4 seconds |
The Item table confirmed that the forces converged at the end of the calculation:
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000002 0.001800 YES RMS Displacement 0.000001 0.001200 YES Predicted change in Energy=-6.142863D-13 Optimization completed. -- Stationary point found.
The calculated vibrational frequencies were all quite low, with the lowest real normal mode being 76.3744:
Low frequencies --- -0.5252 -0.5247 -0.0024 -0.0010 0.0235 1.2010 Low frequencies --- 76.3744 76.3753 99.6982
The vibrational modes of the molecule were animated and are compared in the table below.
| No. | Form of the vibration | Frequency | Intensity | Symmetry (D3h Point Group) |
| 1 | Asymmetric wagging in plane of molecule with two Br atoms moving clockwise whilst the other moves anticlockwise | 76.37 | 3.3447 | E' |
| 2 | Asymmetric stretching with two Br atoms stretching around the Ga atom and the third stretching away from the Ga atom | 76.37 | 3.3447 | E' |
| 3 | Out of plane stretching with Ga stretching perpendicular and the Br atoms stretching symmetrically in the opposite direction | 99.70 | 9.2161 | A2' |
| 4 | Symmetric stretching from all three Br atoms | 197.34 | 0.0000 | A1' |
| 5 | Asymmetric stretching from the Ga atom and two of the Br atoms, with one Br atom still | 316.18 | 57.0704 | E' |
| 6 | Similar to 2 but with no stretching from the two other Br atoms and with stretching from the Ga atom instead | 316.19 | 57.0746 | E' |
The IR spectrum was also attained for GaBr3 from the frequency calculations:

As with the BH3 spectrum there are only three peaks, and for the same reasons. The pairs of vibrations 1 and 2 and vibrations 5 and 6 give one peak each due to the very similar intensities and frequencies, and vibration 4 has no intensity because it is solely a symmetrical stretch from all three outer atoms.
Comparing BH3 and GaBr3 vibrations
| Vibration | BH3 Frequency | BH3 Intensity | BH3 Point Group | GaBr3 Frequency | GaBr3 Intensity | BH3 Point Group |
|---|---|---|---|---|---|---|
| 1 | 1162.99 | 92.5667 | A2' | 76.37 | 3.3447 | E' |
| 2 | 1213.15 | 14.0556 | E' | 76.37 | 3.3447 | E' |
| 3 | 1213.15 | 14.0551 | E' | 99.70 | 9.2161 | A2' |
| 4 | 2582.53 | 0.0000 | A1' | 197.34 | 0.0000 | A1' |
| 5 | 2715.66 | 126.3338 | E' | 316.18 | 57.0704 | E' |
| 6 | 2715.66 | 126.3278 | E' | 316.19 | 57.0746 | E' |
The BH3 vibrations are all at much higher frequencies than GaBr3 vibrations. The larger frequencies are due to the stronger bonds in BH3, discussed above. The only change in terms of the ordering of the molecules is the swap of the A2' vibration, which has the smallest frequency for the BH3 spectrum but the third smallest frequency for the GaBr3 spectrum.
BH3 Molecular Orbitals
Gaussian 09W was used to determine the MOs of BH3 by calculating the energy, with the pop=full keyword. This is the corresponding .log file for the calculation:
An MO diagram[4] was illustrated with the calculated molecular orbitals for BH3:
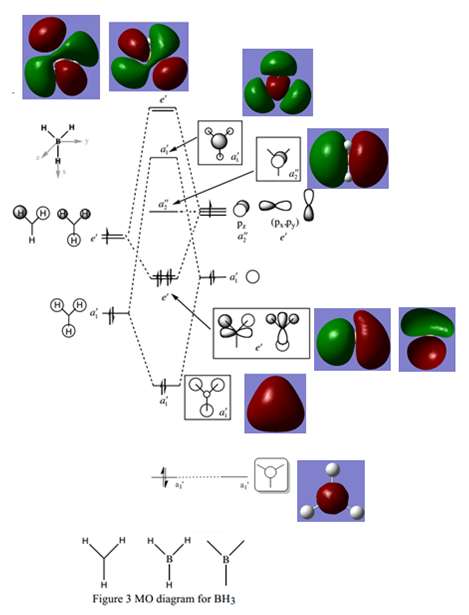
The molecular orbitals as calculated with Gaussian look very similar to the corresponding MOs on the diagram, apart from being more spread out and overlapping where in-phase interactions occur. This suggests that molecular orbital diagrams are very accurate in showing us electron densities for different molecular orbitals and that the theory can give a good idea of bonding in a molecule, even though it is only qualitative.
NBO Analysis
NH3 Optimisation
Gaussian 09W was used to calculate the optimisation of NH3:
This is the link to the .log file:
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Charge | 0 |
| Spin | Singlet |
| Final Energy | -56.55776873 a.u. |
| Gradient | 0.00000323 a.u. |
| Dipole Moment | 1.8465 D |
| Point Group | C3V |
| Calculation Time | 1 minute 30.0 seconds |
The .log file confirmed that the calculation had converged:
Item Value Threshold Converged? Maximum Force 0.000006 0.000015 YES RMS Force 0.000004 0.000010 YES Maximum Displacement 0.000012 0.000060 YES RMS Displacement 0.000008 0.000040 YES Predicted change in Energy=-9.846372D-11 Optimization completed. -- Stationary point found.
| Name | Value | Value |
|---|---|---|
| R1 | R(1,2) | 1.018 |
| R2 | R(1,3) | 1.018 |
| R3 | R(1,4) | 1.018 |
| A1 | A(2,1,3) | 105.7446 |
| A2 | A(2,1,4) | 105.7446 |
| A3 | A(3,1,4) | 105.7446 |
| D | D(2,1,4,3) | -111.8637 |
NH3 Frequency
Gaussian 09W was used to calculate the frequency of NH3:
This is the link to the .log file:
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Charge | 0 |
| Spin | Singlet |
| Final Energy | -56.55776872 a.u. |
| Gradient | 0.00000322 a.u. |
| Dipole Moment | 1.8465 D |
| Point Group | C3 |
| Calculation Time | 17.4 seconds |
The .log file showed that the calculation had converged:
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.000013 0.001800 YES RMS Displacement 0.000007 0.001200 YES Predicted change in Energy=-1.131338D-10 Optimization completed. -- Stationary point found.
There were however some very slightly negative frequencies:
Low frequencies --- -0.0139 -0.0035 -0.0010 7.0781 8.0927 8.0932 Low frequencies --- 1089.3840 1693.9368 1693.9368
The vibrational modes of the molecule were animated and are compared in the table below.
| No. | Form of the vibration | Frequency | Intensity | Symmetry (D3h Point Group) |
| 1 | All three H atoms waggle symmetrically with the N atom still | 1089.38 | 145.4273 | A1 |
| 2 | Asymmetric waggling, with two H atoms waggling together and the third asymmetrically to them | 1693.94 | 13.5570 | E |
| 3 | Symmetric waggling from two of the H atoms, with the third moving up and down | 1693.94 | 13.5571 | E |
| 4 | Symmetric stretching from all three H atoms | 3461.30 | 1.0595 | A1 |
| 5 | Anti-symmetric stretching from just two of the H atoms, with the final H atom still | 3589.86 | 0.2699 | E |
| 6 | Asymmetric stretching with two H atoms stretching at a different time to the third one | 3589.86 | 0.2699 | E |
The IR spectrum was also calculated for the NH3 molecule:

There only appear to be two peaks in this IR. The peak with the largest intensity corresponds to the first vibration, the second peak corresponds to the second and third peaks, each with the same frequency, and there is a group of the other peaks at the other end of the spectrum, with very low intensities compared to the first three vibrations.
Gaussian 09W was used to calculate the population analysis (MOs) of NH3:
This is the link to the .log file:
Association Energies
NH3BH3 Optimisation
Gaussian 09W was used to calculate the optimisation of NH3BH3:
This is the link to the .log file:
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Charge | 0 |
| Spin | Singlet |
| Final Energy | -83.22468906 a.u. |
| Gradient | 0.00000124 a.u. |
| Dipole Moment | 5.5646 D |
| Point Group | C1 |
| Calculation Time | 2 minutes 49.6 seconds |
The .log file converged:
Item Value Threshold Converged? Maximum Force 0.000002 0.000015 YES RMS Force 0.000001 0.000010 YES Maximum Displacement 0.000026 0.000060 YES RMS Displacement 0.000009 0.000040 YES Predicted change in Energy=-8.958526D-11 Optimization completed. -- Stationary point found.
NH3BH3 Frequency
Gaussian 09W was used to calculate the frequency of NH3BH3:
This is the link to the .log file:
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Charge | 0 |
| Spin | Singlet |
| Final Energy | -83.22468912 a.u. |
| Gradient | 0.00000117 a.u. |
| Dipole Moment | 5.5646 D |
| Point Group | C1 |
| Calculation Time | 1 minute 56.9 seconds |
The .log file converged:
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000022 0.001800 YES RMS Displacement 0.000012 0.001200 YES Predicted change in Energy=-9.661898D-11 Optimization completed. -- Stationary point found.
There were again small negative charges found in the low frequencies:
Low frequencies --- -1.0359 -0.0008 -0.0005 0.0004 3.5646 4.4382 Low frequencies --- 263.4534 632.9697 638.4506
References
- ↑ 1.0 1.1 Haynes, W.M., CRC Handbook of Chemistry and Physics 93rd Edition, CRC Press, Taylor & Francis Group, 2012, 9-21.
- ↑ Haynes, W.M., CRC Handbook of Chemistry and Physics 93rd Edition, CRC Press, Taylor & Francis Group, 2012, 9-23.
- ↑ Haynes, W.M., CRC Handbook of Chemistry and Physics 93rd Edition, CRC Press, Taylor & Francis Group, 2012, 9-20.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedBH3 MO Diagram
