Rep:Mod:IR616
NH3 Molecule
Optimisation
| Calculation Method | RB3LYP |
| Basis Set | CC-pVDZ |
| Final Energy (au) | -56.55775873 |
| RMS Gradient (au) | 0.00000323 |
| Point Group | C3V |
| Bond Length | 1.02 |
| Bond Angle | 105.7 |
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000004 0.001800 YES RMS Displacement 0.000009 0.001200 YES
Media:IROBINSON_AMMONIA_OPTIMISATION.LOG
Interactive Diagram
ammonia |
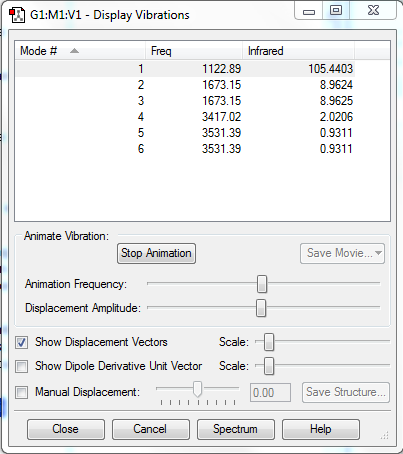
Vibrations
| Number of Modes | 6 |
| Degenerate Modes | (2&3) (5&6) |
| Bending Vibrations | 1,2,3 |
| Stretching Vibrations | 4,5,6 |
| Highly Symmetric Mode | 4 |
| Umbrella Mode | 1 |
| Number of bands in an experimental spectrum | 4 |
Charge
| Nitrogen | -1.048 |
| Hydrogen | 0.349 |
Nitrogen is expected to have a negative partial charge whilst hydrogen is expected to have a positive partial charge, because nitrogen is more electronegative than hydrogen so attracts the electrons in the covalent bonds more strongly.
N2 Molecule
Optimisation
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy (au) | -109.52412868 |
| RMS Gradient (au) | 0.00000060 |
| Point Group | D*H |
| Bond Length | 1.11 |
| Bond Angle | 180 |
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
Interactive Diagram
ammonia |
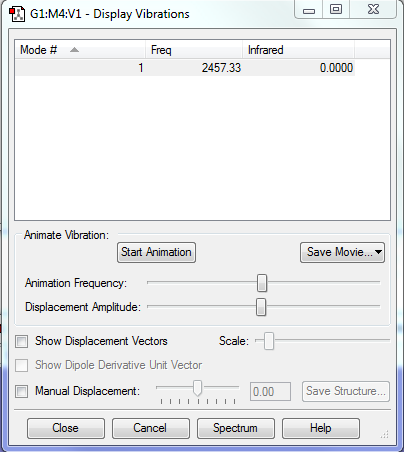
Vibrations
There is only one vibration, because the molecule is linear and diatomic (3N-5).
H2 Molecule
Optimisation
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy (au) | -1.17853935 |
| RMS Gradient (au) | 0.00003809 |
| Point Group | D*H |
| Bond Length | 0.743 |
| Bond Angle | 180 |
Item Value Threshold Converged? Maximum Force 0.000066 0.000450 YES RMS Force 0.000066 0.000300 YES Maximum Displacement 0.000087 0.001800 YES RMS Displacement 0.000123 0.001200 YES
Interactive Diagram
ammonia |
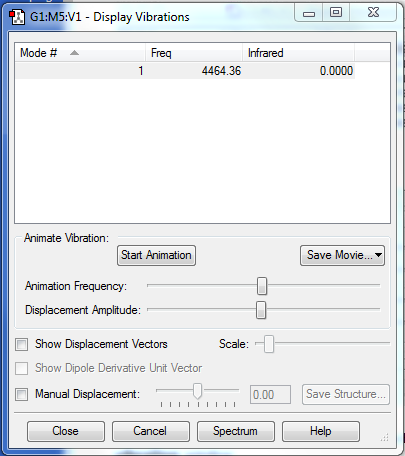
Vibrations
There is only one vibration, because the molecule is linear and diatomic (3N-5).
N2 + 3H2 → 2NH3
Reaction Energies (au)
| E(NH3) | -56.55776873 |
| 2*E(NH3) | -113.1155375 |
| E(N2) | -109.52412868 |
| E(H2) | -1.17853935 |
| 3*E(H2) | -3.53561805 |
| ΔE=2*E(NH3)-[E(N2)+3*E(H2)] | -0.05579073 |
Overall Reaction Energy (KJmol-1)
| ΔE | -146.48 |
| Sum of energies of reactants | Energy of product |
| -296838 | -296966 |
The energy of ammonia is lower than the energies of the molecules from which it was formed, therefore it is more stable than the gaseous reactants.
ClF
Optimisation
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy (au) | -559.94269578 |
| RMS Gradient (au) | 0.00014211 |
| Point Group | C*V |
| Bond Length | 1.66434 |
| Bond Angle | 180 |
Item Value Threshold Converged? Maximum Force 0.000246 0.000450 YES RMS Force 0.000246 0.000300 YES Maximum Displacement 0.000433 0.001800 YES RMS Displacement 0.000613 0.001200 YES
Interactive Diagram
ammonia |
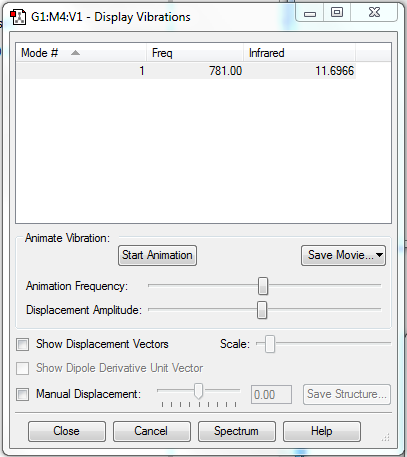
Vibrations
There is only one vibration, because the molecule is linear and diatomic (3N-5). Here the vibration won't be even, because the chlorine atom is heavier, so will move a shorter distance than the fluorine atom from the point of equilibrium.
Charge
| Chlorine | 0.309 |
| Flurorine | -0.309 |
Fluorine is more electronegative than chlorine, so has a negative partial charge whilst chlorine's is positive.
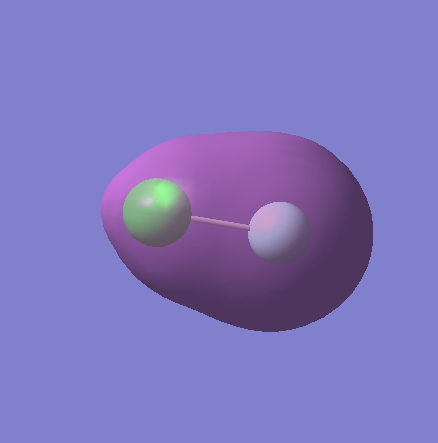
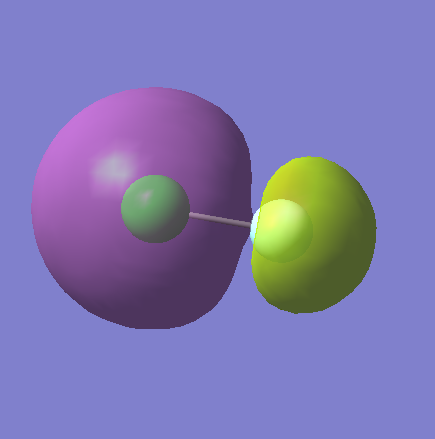
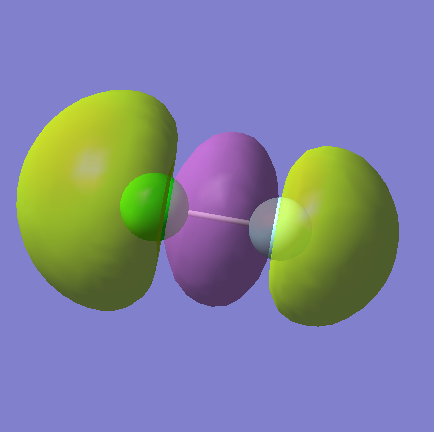
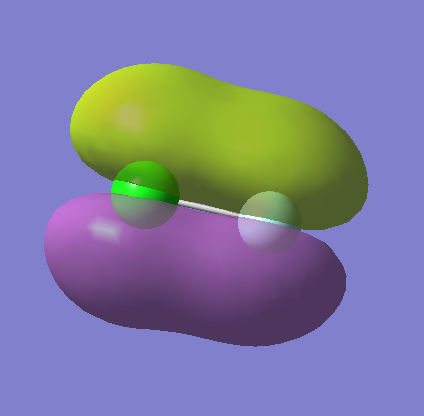
Molecular Orbitals
MO7
This is the overlap of the bonding 3s AO from chlorine and the 2s from fluorine. It is occupied and is fairly deep in energy at -1.21864, and is the deepest out of the molecular orbitals, however not as deep as the non-bonding atomic orbitals.
MO8
This is the combination of the 3s AO from the chlorine and the 2s from the fluorine. It is an antibonding orbital and it is occupied. It is higher in energy at -0.83311.
MO9
This is the combination of the 3p from the chlorine and the 2p from the fluorine. This is an bonding orbital and it is occupied. It is again higher in energy at -0.52314.
MO10
This is the overlap of the 3p and 2p AOs. It is bonding and occupied. It is yet still higher in energy at -0.46713.
MO12
This is another combination of the 3p AO from chlorine and the 2p AO from fluorine, but this is antibonding and occupied. It is in the HOMO/LUMO region as the HOMO of the molecule at the energy -0.32855, meaning it is the highest of the occupied energy levels.
Cl2
Optimisation
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy (au) | -920.34987886 |
| RMS Gradient (au) | 0.00002511 |
| Point Group | D*H |
| Bond Length | 2.04 |
| Bond Angle | 180 |
Cl2 has a much lower energy than CLF, so is more stable.
Item Value Threshold Converged? Maximum Force 0.000043 0.000450 YES RMS Force 0.000043 0.000300 YES Maximum Displacement 0.000121 0.001800 YES RMS Displacement 0.000172 0.001200 YES
Interactive Diagram
chlorine |
Vibrations
There is only one vibration, because the molecule is linear and diatomic (3N-5).
Charge
| Chlorine | 0 |
There is no difference in electronegativity between the two atoms, because they are the same element, hence why neither has a partial charge. This is different from ClF.