Rep:Mod:IM915
Revision of Core Concepts and Introduction to Basis Sets
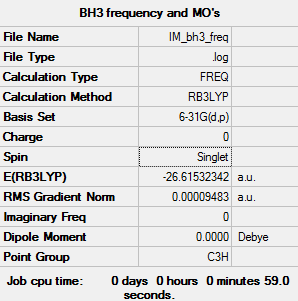
Borane: BH3
Key data
This calculation was carried out using the B3LYP/6-31G(d,p) basis set
Item Table
Item Value Threshold Converged? Maximum Force 0.000190 0.000450 YES RMS Force 0.000095 0.000300 YES Maximum Displacement 0.000747 0.001800 YES RMS Displacement 0.000374 0.001200 YES
Log file for Borane (Frequency file)
Frequencies
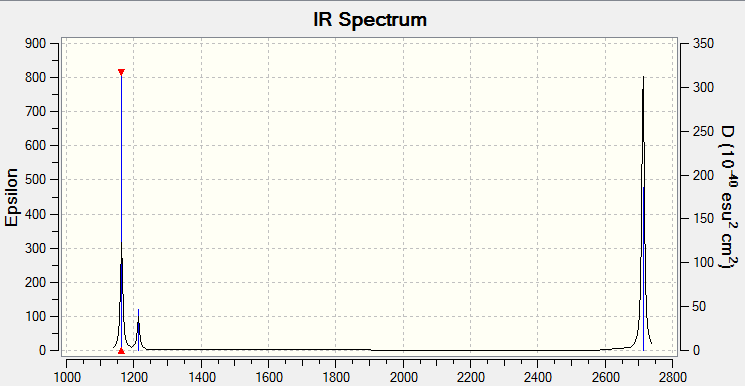
Low frequencies --- -0.2260 -0.1036 -0.0055 48.0278 49.0875 49.0880 Low frequencies --- 1163.7224 1213.6715 1213.6741
Borane |
Vibrational spectrum for BH3
| wavenumber (cm-1) | Intensity (arbitrary units) | symmetry | IR active? | Type |
| 1164 | 92 | A'' | yes | out-of-plane bend |
| 1214 | 14 | E' | very slight | bend |
| 1214 | 14 | E' | very slight | bend |
| 2580 | 0 | A' | no | symmetric stretch |
| 2713 | 126 | E' | yes | asymmetric stretch |
| 2713 | 126 | E' | yes | asymmetric stretch |
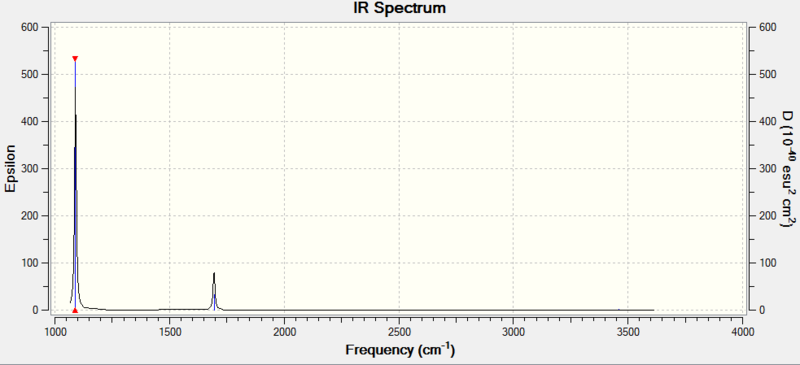
Predicted IR for Borane
While there are 6 vibrations possible for the molecule (listed above), only 5 of these are IR active- the symmetric stretch is not IR active as it cannot result in a change of dipole across the molecule. There are 5 IR active vibrations, however only 3 peaks can be seen in the spectrum. This is due to degenerate vibrations- there are 2 degenerate bends (E') at 1214 cm-1 which appear as a single peak in the IR, and two degenerate asymmetric stretches (E') at 2713 cm-1 which also appear as a single peak in the IR. This explains why only 3 peaks are predicted in the IR spectrum.
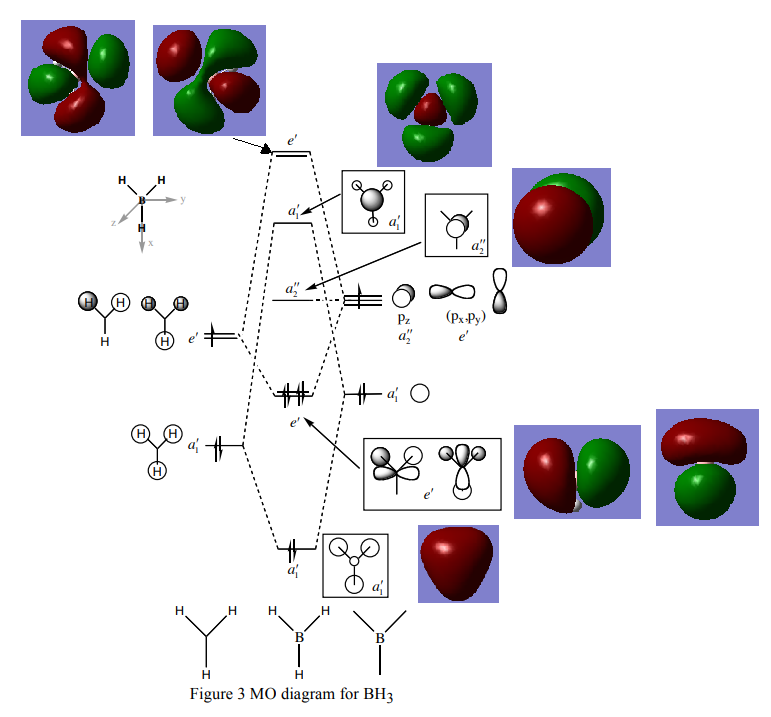
Borane MO diagram and calculated MO's.
LCAO MO Diagram[1]
The computed MO's are seen to be almost identical to the LCAO MO's, meaning that for this small molecule, and likely for other small molecules, a qualitative approach to MO theory is adequate to describe the system to a high degree of accuracy.
Smf115 (talk) 16:31, 26 May 2018 (BST)Nice inlcusion of the MOs however, the top e' one seems to have the same MO twice (with phases switched) and so one is missing. The similarity of the LCAOs is highlighted but to improve, consider the subtle differences in some of the orbital contributions in some of the MOs, especially 3a1'.
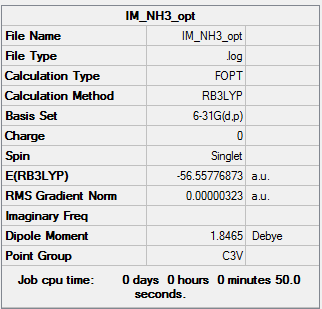
Ammonia: NH3
Key data
This calculation was carried out using the B3LYP/6-31G(d,p) basis set
Item Table
Item Value Threshold Converged? Maximum Force 0.000013 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000039 0.001800 YES RMS Displacement 0.000013 0.001200 YES
Log file for Ammonia (Frequency file)
Frequencies
Low frequencies --- -8.5646 -8.5588 -0.0044 0.0454 0.1784 26.4183 Low frequencies --- 1089.7603 1694.1865 1694.1865
Ammonia |
Vibrational spectrum for NH3
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | Type |
| 1090 | 145 | A | yes | bend |
| 1694 | 14 | E | very slight | bend |
| 1694 | 14 | E | very slight | bend |
| 3461 | 1 | A | no | symmetric stretch |
| 3589 | 0 | E | no | asymmetric stretch |
| 3589 | 0 | E | no | asymmetric stretch |
Predicted IR for Ammonia
Similarly to Borane, there are 6 vibrations possible. Only 3 are IR active in ammonia, two of which are degenerate, resulting in 2 peaks in the IR spectrum.
Ammonia-Borane: BH3NH3
Key data
This calculation was carried out using the B3LYP/6-31G(d,p) basis set
Item Table
Item Value Threshold Converged? Maximum Force 0.000116 0.000450 YES RMS Force 0.000063 0.000300 YES Maximum Displacement 0.000797 0.001800 YES RMS Displacement 0.000359 0.001200 YES
Log file for Ammonia-Borane (Frequency file)
Frequencies
Low frequencies --- -0.0003 0.0005 0.0007 15.2004 18.7944 42.3939 Low frequencies --- 266.2666 632.2878 639.1356
Ammonia-Borane |
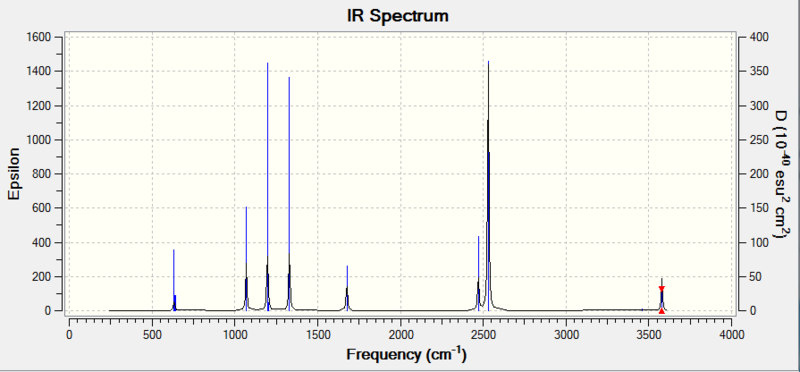
Vibrational spectrum for NH3BH3
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | Type |
| 266 | 0 | A | no | Torsion |
| 632 | 14 | A | slight | stretch |
| 639 | 4 | A | very slight | rocking |
| 640 | 4 | A | very slight | bend |
| 1069 | 41 | A | yes | bend |
| 1069 | 40 | A | yes | bend |
| 1197 | 40 | A | yes | out-of-plane asymmetric bend |
| 1204 | 4 | A | very slight | bend |
| 1330 | 114 | A | yes | out-of-plane asymmetric bend |
| 1676 | 28 | A | slight | bend |
| 1677 | 28 | A | slight | bend |
| 2470 | 67 | A | yes | symmetric stretch |
| 2530 | 231 | A | yes | asymmetric stretch |
| 2530 | 231 | A | yes | asymmetric stretch |
| 3462 | 2.5 | A | very slight | symmetric stretch |
| 3579 | 28 | A | slight | asymmetric stretch |
| 3579 | 28 | A | slight | asymmetric stretch |
Predicted IR for Ammonia-Borane
Ammonia-Borane N-B Bond Energy
E(NH3)= -56.55777 a.u. E(BH3)= -26.61532 a.u. E(NH3BH3)= -83.22469 a.u.
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)] = -83.22469 - [-56.55777 -26.61532] = -0.05160 a.u. = -135.5 kJmol-1
This is a reasonable value for a bond, and is a weaker bond than both a N-N single bond (167 kJmol-1) and a P-P single bond (201 kJmol-1). Dative covalent bonds are weaker bonds than non-dative covalent bonds, and have a high polarity. As N is far more electronegative than B, this bond is highly polar and the majority of electron density will be located on nitrogen, as shown in the charge analysis (see section 1.3.4).
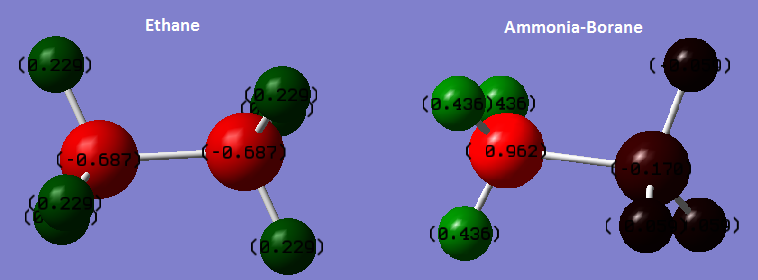
Comparison with Ethane: NBO Charges
| Atom | Charge (arbitrary units) |
|---|---|
| C | –0.687 |
| H | 0.229 |
| Atom | Charge (arbitrary units) |
|---|---|
| N | –0.962 |
| B | –0.170 |
| HN | 0.436 |
| HB | –0.059 |
In ethane, both carbon atoms have the same charge (-0.687) and all hydrogen atoms have the same charge (0.229). This is due to the symmetry of the molecule, as both carbons are in identical environments, as are all 6 hydrogens. Carbon is more electronegative than hydrogen so most of the electron density will be on carbon - reflected by the negative charge on carbon.
In Ammonia-Borane the symmetry is lost due to the difference between the nitrogen and boron atoms. This also results in different environments for the hydrogen atoms on the ammonia fragment and the borane fragment. Nitrogen is far more electronegative than boron, and so the majority of electron density sits on nitrogen, resulting in the larger magnitude of negative charge on nitrogen (-0.962). This also causes the hydrogens on the ammonia fragment to have a larger magnitude of positive charge (0.436). While boron is more electronegative than hydrogen, the difference is small, and the magnitude of negative charge on the boron is already reduced due to the high electronegativity of nitrogen. This results in only very slight negative charges on boron and the hydrogen atoms on the borane fragments. This molecule will have an overall linear dipole, as the only positive atoms are the hydrogen atoms in the ammonia fragment while the rest of the atoms in the molecule are negative.
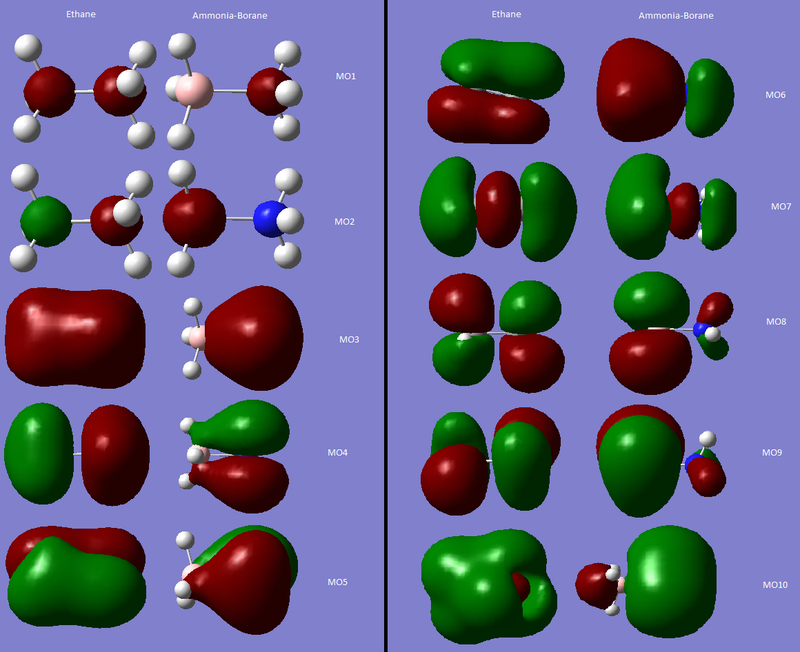
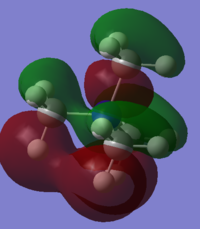
Comparison with Ethane: Molecular orbitals
Shown are the Molecular Orbitals for both Ethane and Ammonia-Borane. In Ethane, the molecular orbitals are symmetric through the C-C bond as the molecule has a mirror plane through this bond. In Ammonia-Borane this symmetry is broken by having two different atoms (N-C). The difference in electronegativities of these atoms means the atomic orbitals associated with the NH3 and BH3 fragments will be at different energies, resulting in non-symmetric molecular orbitals. Nitrogen is more electronegative than Boron, therefore the atomic orbitals of the ammonia fragment will be lower in energy (more negative). This will mean that the bonding atomic orbitals will have a greater contribution from the ammonia fragment, whereas antibonding orbitals will have a greater contribution from the borane fragment. This can be seen clearly in MO3, which is totally bonding- in ammonia-borane there is far more electron density on the ammonia fragment. In contrast, in MO8, an antobonding MO, there is far more electron density on the borane fragment.
Smf115 (talk) 16:32, 26 May 2018 (BST)Excellent charge analysis and MO comparion between ethane and NH3BH3! Clearly explained points and figures.
Boron Tribromide: BBr3
Key data
This calculation was carried out using the B3LYP/6-31G(d,p) basis set (for Boron) and LANL2DZ pseudo potential (for Bromine)
Database entry can be found at DOI:10042/202413
Item Table
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000036 0.001800 YES RMS Displacement 0.000018 0.001200 YES
Log file for Boron Tribromide (Frequency file)
Frequency
Low frequencies --- -0.0137 -0.0064 -0.0047 2.4315 2.4315 4.8421 Low frequencies --- 155.9631 155.9651 267.7052
Boron tribromide |
Vibrational spectrum for BBr3
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | Type |
| 156 | 0 | E' | no | bend |
| 156 | 0 | E' | no | bend |
| 268 | 0 | A1' | no | symmetric stretch |
| 378 | 4 | A2'' | very slight | out-of-plane bend |
| 763 | 320 | E' | yes | asymmetric stretch |
| 763 | 320 | E' | yes | asymmetric stretch |
Predicted IR for BBr3
Project: Ionic Liquids
Tetramethylammonium Ion ([N(CH3)4]+): Key Data
This calculation was carried out using the B3LYP/6-31G(d,p) basis set.
Item Table
Item Value Threshold Converged? Maximum Force 0.000062 0.000450 YES RMS Force 0.000021 0.000300 YES Maximum Displacement 0.000399 0.001800 YES RMS Displacement 0.000153 0.001200 YES
Log file for the Tetramethylammonium Ion (Frequency file)
Frequencies
Low frequencies --- -5.3709 0.0006 0.0009 0.0010 4.9091 7.9341 Low frequencies --- 182.2339 287.9158 288.6033
While there is a negative frequency (which would normally indicate a failed optimisation), in this case as it is a very small negative number this instead can be ascribed to the basis set used being not fully adequate for this system.
Tetramethylammonium Ion |
Smf115 (talk) 13:31, 27 May 2018 (BST)Good structure information and inclusion of the charge on both molecules. However, the negative frequency is one of the low frequency modes relating to translational and rotational modes of the molecule (the 6 from the 3N-6 formula) and it is normal for these to be negative. You should be aware of the magnitude of these 6 low frequencies when doing calculations though.
Tetramethylphosphonium Ion ([P(CH3)4]+): Key data
This calculation was carried out using the B3LYP/6-31G(d,p) basis set.
Item Table
Item Value Threshold Converged? Maximum Force 0.000126 0.000450 YES RMS Force 0.000033 0.000300 YES Maximum Displacement 0.000845 0.001800 YES RMS Displacement 0.000304 0.001200 YES
Log file for the Tetramethylphosphonium Ion
Frequencies
Low frequencies --- -0.0037 -0.0034 -0.0033 8.9413 11.8632 15.1417 Low frequencies --- 157.1005 192.3734 192.6565
While there are negative frequencies (which would normally indicate a failed optimisation), in this case as they are very small negative numbers this instead can be ascribed to the basis set used being not fully adequate for this system.
Tetramethylphosphonium Ion |
NBO Charge Comparison
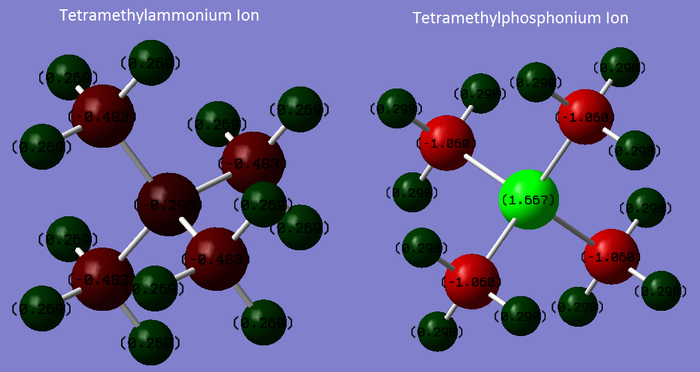
|
| Atom | Charge (arbitrary units) |
|---|---|
| N | –0.295 |
| C | –0.483 |
| H | 0.269 |
| Atom | Charge (arbitrary units) |
|---|---|
| P | 1.667 |
| C | –1.060 |
| H | 0.298 |
The hydrogen atoms in both ions have a similar charge (0.269 for [N(CH3)4]+ and 0.298 for [P(CH3)4]+), as they are two bonds away from the atom that differs between these two molecules and are therefore similar in environment. There is a total of 3.23 units of positive charge located on the hydrogen atoms in [N(CH3)4]+, while there is a total of 3.58 units of positive charge located on the hydrogen atoms in [P(CH3)4]+. There is therefore slightly less electron density located on the hydrogen atoms in [P(CH3)4]+, i.e. there is a greater surface positive charge in [P(CH3)4]+ (as the hydrogen atoms are effectively the surface of the ion).
The Carbon atoms in both ions are negatively charged, as carbon is more electronegative than hydrogen and electron density in the C-H bonds will be drawn towards C. The carbons in [P(CH3)4]+ are significantly more negative than those in [N(CH3)4]+ (with over twice the charge). This is due to the difference in central atom electronegativity described next.
The most interesting difference between the ions is the charge on the central atom. In [N(CH3)4]+, the central N atom is negative as nitrogen is more electronegative than carbon, therefore electron density in the N-C bonds will be drawn towards the nitrogen. This reduces the magnitude of the negative charge on carbon atoms in [N(CH3)4]+. However in [P(CH3)4]+, the central P atom is significantly positive (with the largest magnitude of charge seen on any atom in either ion). This is due to the difference in electronegativity- being a third row element Phosphorus is far less electronegative than Nitrogen, and in contrast to Nitrogen is less electronegative than Carbon. This means electron density in the P-C bonds will instead be drawn towards the Carbon atoms, resulting in a positive charge on Phosphorus and a negative charge of a higher magnitude on Carbon.
Tetraalkylammonium Ion
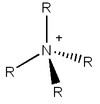
[NR4]+ (where R is an alkyl group) is commonly depicted with the positive charge on the nitrogen atom, as shown. However, this is a misleading description of the ion. The positive charge is placed on nitrogen as it has 4 bonds, as opposed to it's neutral standard valence with 3 bonds and a lone pair. Under a classical interpretation, this would result in only 4 electrons 'belonging' to nitrogen, as opposed to 5, resulting in a positive charge.
However, my charge analysis shows that for [N(CH3)4]+ the central itrogen atom is in fact negative, as are the carbons bonded to nitrogen- the positive charge in the cation is actually located on the hydrogen atoms of the methyl groups. This can be extrapolated to all [NR4]+ ions - the positive charge in fact sits on the hydrogens of the alkyl chains.
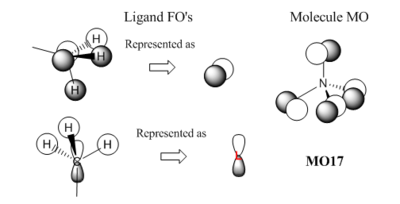
[N(CH3)4]+ Molecular Orbitals
From the molecular orbital calculations it can be determined that the first valence orbital is MO6, as MO5 has a significantly lower energy of -10.4143 atomic units, while MO6 has an energy of -1.1964 atomic units. This would indicate that orbitals 1-5 are core orbitals formed from the Nitrogen and Carbon 1s orbitals (4 C 1s, 1 N 1s), and can therefore be ignored in bonding analysis. The filled valence orbitals that contribute to bonding in the molecule are therefore MO6 - MO21, while MO22 is the LUMO of this system.
Smf115 (talk) 13:52, 27 May 2018 (BST)Good range of MOs selected and nice mention of the difference between the valence and core orbitals. Overall the LCAOs are largely correct however, some of the FOs aren't completely right and there seems to be several ligand FOs for MO 17 but only one used and a mistake has been made labelling the MO name. To improve, consider the BH3 fragments, seen both here and in your lectures, which can be used for the CH3 groups. Nice additional analysis of the MOs and the conributions.
Smf115 (talk) 13:52, 27 May 2018 (BST)Overall a clearly presented and good wiki report.
- ↑ Dr. P Hunt, Molecular Orbitals Lecture Course, Imperial College London, November, 2017.