Rep:Mod:IGE15IMM2
Practice Molecules
NH3 Optimisation
| Calculation Method | RB3LYP |
|---|---|
| Basis Set | 6-31G(d,p) |
| E(RB3LYP) /a.u. | -56.55776873 |
| RMS Gradient /a.u. | 0.00000485 |
| Point Group | C3V |
| N-H Bond length /Å | 1.01798 |
| H-N-H Bond angle /° | 105.741 |
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
Optimised Ammonia Molecule |
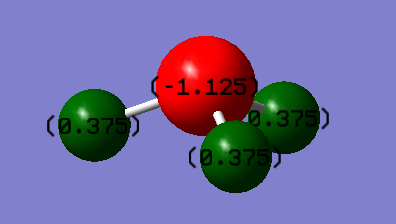
N is more electronegative than H and so we expect it to have a negative charge, whilst H should have a positive charge.
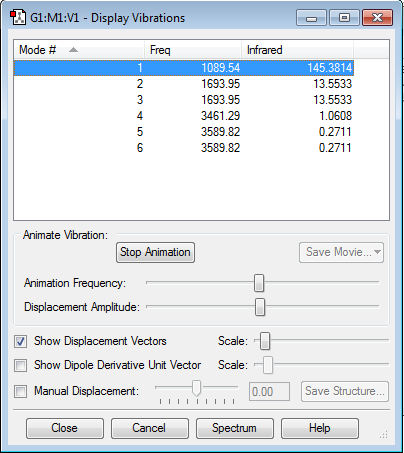
Vibrations
We expect 6 modes of vibration.
2/3 and 5/6 are degenerate.
1,2,3 are bending, 4,5,6 are stretch vibrations.
4 is highly symmetric.
1 is known as the "umbrella" mode.
4 bands.
N2 Optimisation
| Calculation Method | RB3LYP |
|---|---|
| Basis Set | 6-31G(d,p) |
| E(RB3LYP) /a.u. | -109.52412868 |
| RMS Gradient /a.u. | 0.00000060 |
| Point Group | D∞H |
| N-N Bond length /Å | 1.10550 |
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
Optimised Nitrogen Molecule |
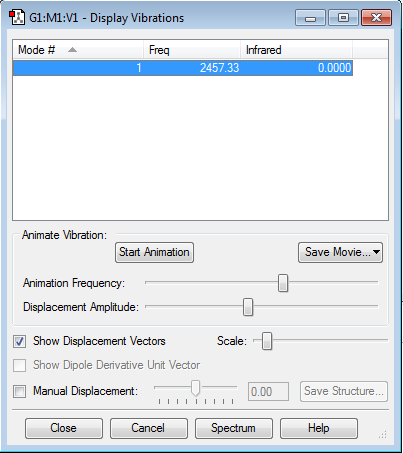
Vibrations
As a homonuclear diatomic molecule, there is no charge on each atom. There is 1 mode of vibration, with no change in dipole moment and so it is not IR active.
H2 Optimisation
| Calculation Method | RB3LYP |
|---|---|
| Basis Set | 6-31G(d,p) |
| E(RB3LYP) /a.u. | -1.17853936 |
| RMS Gradient /a.u. | 0.00000017 |
| Point Group | D∞H |
| H-H Bond length /Å | 0.74279 |
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
Optimised Hydrogen Molecule |
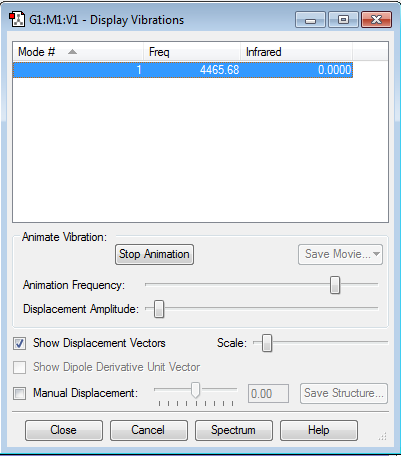
Vibrations
As with N2, the atoms are uncharged and the single mode of vibration is not IR active.
Haber-Bosch Process
E(NH3) = -56.55776873
2*E(NH3) = -113.11553746
E(N2) = -109.52412868
E(H2) = -1.17853936
3*E(H2) = -3.53561808
ΔE=2*E(NH3)-[E(N2)+3*E(H2)] = -0.0557907 a.u. = -146.48 kJ/mol
The reaction is exothermic and so ammonia is more stable than the gaseous reactants.
Molecule of Choice: SiH4
Optimisation
| Calculation Method | RB3LYP |
|---|---|
| Basis Set | 6-31G(d,p) |
| E(RB3LYP) /a.u. | -291.88802760 |
| RMS Gradient /a.u. | 0.00000002 |
| Point Group | TD |
| Si-H Bond Length /Å | 1.48485 |
| H-Si-H Bond Angle /° | 109.471 |
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
Optimised Silane Molecule |

As Si has an electronegativity of 1.9 compared to 2.2 of H, there is a slight positive charge on the Si and negative charges on the H atoms.
Vibrations
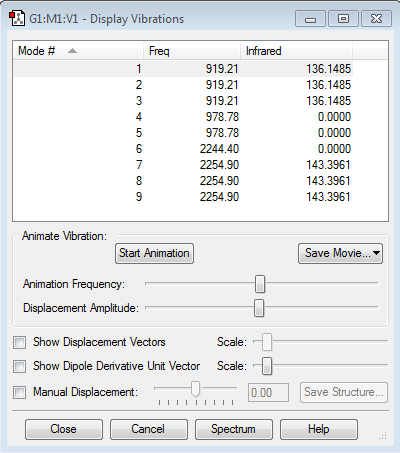
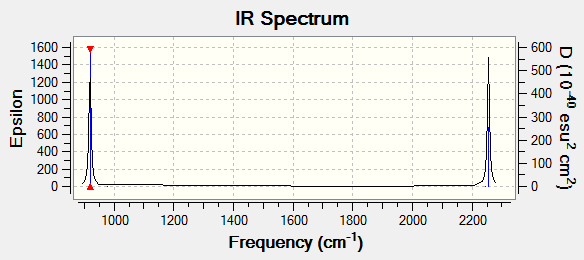
As can be seen in the calculated spectrum, there are only 2 peaks, each for 3 degenerate modes. The other 3 modes, #4,5 and 6, are not seen in IR spectrum as there is no change in dipole moment.
Molecular Orbitals
Comparison with CH4
CH4 Optimisation
| Calculation Method | RB3LYP |
|---|---|
| Basis Set | 6-31G(d,p) |
| E(RB3LYP) /a.u. | -40.52401404 |
| RMS Gradient /a.u. | 0.00003263 |
| Point Group | TD |
| C-H Bond Length /Å | 1.09197 |
| H-C-H Bond Angle /° | 109.471 |
Item Value Threshold Converged? Maximum Force 0.000063 0.000450 YES RMS Force 0.000034 0.000300 YES Maximum Displacement 0.000179 0.001800 YES RMS Displacement 0.000095 0.001200 YES
Optimised Methane Molecule |

While Si is less electronegative than H, C at 2.55 is more electronegative than H and so has a negative charge while the H atoms have positive charge.
Vibrations
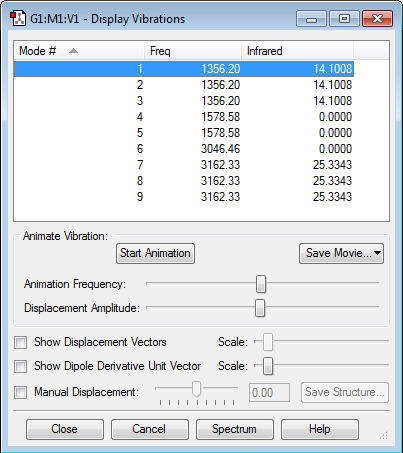
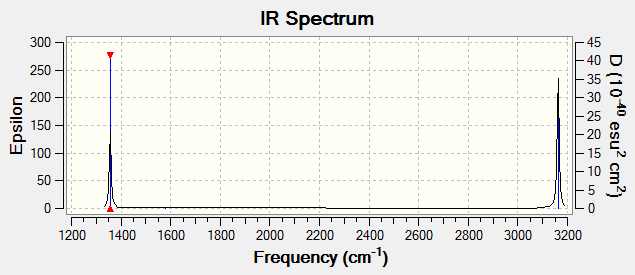
The C-H bond is stronger than the Si-H bond as evidenced by its shorter length. As a result, although CH4 and SiH4 have the same tetrahedral symmetry and so the same modes of vibration, the CH4 vibrations have higher energy and are found at higher frequencies.
C forms stronger bonds with H than Si does because its sp3 orbitals are smaller and have a better overlap with the H 1s orbital. In addition, there is a larger electronegativity difference and so more ionic character.