Rep:Mod:Hayley Weir Transition states and reactivity2
This computation lab aims to explore the [[ https://en.wikipedia.org/wiki/Cope rearrangement|Cope Rearrangement]] and the Diels-Alder Reaction reaction pathways and transition states structures. Both reactions are examples of pericyclic reactions, a class of reaction in which there is a cyclic transition state and the reaction is concerted. There are 3 main types of pericyclic reactions: electrocyclic reactions, cycloaddition and sigmatropic rearrangements.
Electrocyclic reaction is "A molecular rearrangement that involves the formation of a σ-bond between the termini of a fully conjugated linear π-electron system (or a linear fragment of a π-electron system) and a decrease by one in the number of π-bonds, or the reverse of that process."[1]
A cycloaddition reaction consits of "two or more unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." [2]
A sigmatropic rearangement is "A molecular rearrangement that involves both the creation of a new σ-bond between atoms previously not directly linked and the breaking of an existing σ-bond"[3]
The Woodward Hoffman rules determine whether a pericyclic reaction will happen or not. The rule states:
'In allowed thermal pericyclic reactions the number of (4q+2)s and (4r)a components must be odd.'[4]
This rule is reversed for photochemical reactions. In this lab thermal reactions are considered. q and r are integers and correspond to the number of suprafacial (s) components and antarafacial (a) components respectively.

The Cope rearrangement is classified as a [3,3] sigmatropic reaction. The numbers [i,j] correspond to the number of atoms between the bond breaking and bond forming in both directions of the reactions.This system is pi2a +pi2s + sig2a or pi2s +pi2s + sig2s as shown below.
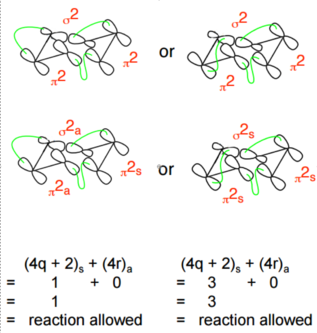
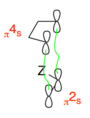
Applying Woodward Hoffman analysis to this system: pi2s counts as 1 towards the (4q +2)s component when q = 0 and pi2a counts as 0 towards both components. Overall this gives (1)s + (0)a = 1 or (3)s + (0)a = 3 which are both odd, therefore this reaction is thermally allowed.
The Diels-alder reaction is classified as a [4+2] cycloaddition. The [i+j] numbering represents the number of atoms in the pi systems reacting. This system is pi4s + pi2s. Applying Woodward Hoffman analysis to this system: pi4s counts as 0 towards both components as it does not satisfy either, and pi2s counts as 1 towards the (4q +2)s component when q = 0. Overall this gives (1)s + (0)a = 1 which is odd, therefore this reaction is thermally allowed.
The Cope Rearrangement Tutorial
Optimizing the Reactants and Products
Aims of section:
- Optimize structure
- Determine point group
- Calculate vibrational frequencies and visualize
- Calculate potential energies to compare them with experimental values
- Determine preferred reaction mechanism
Anti-periplanar 1,5 - cyclohexadiene
The structures in table 1 were optimized at the HF/3-21G level of theory. HF refers to Hartree Fock, and 3-21G is the lowest level of theory.
| Conformer | Structure | Calculation | Energy/Hartrees | Point group | Comment | |
|---|---|---|---|---|---|---|
| a) |
Anit periplanar 1,5 hexadiene |
 |
HF/3-21G | -231.69097054 | C1 | |
| b) |
Gauche 1,5 hexadiene |
 |
HF/3-21G | Prediction: Higher energy than app due to steric clash
Result: -231.68961573 Prediction correct |
C1 | |
| ci) |
Anti1 (lowest energy conformer) [7] |
 |
HF/3-21G | -231.69260231 | C2 | Lowest energy conformer expected to have *****COME BACK TO****
From table it can be seen that anti1 has the lowest energy |
| cii) | Gauche3 |  |
HF/3-21G | -231.69266116 | C1 | Gauche3 is the lowest conformer because *****COME BACK TO**** |
| d) |
Comparision:Anit periplanar 1,5 hexadiene corresponds to anti4 in the appandix [7] and Gauche 1,5 hexadiene corresponds to gauche5[7] | |||||
| e) |
Anti2 |
 |
HF/3-21G | -231.69253520
cf -231.69254 from table [7] |
Ci | |
f) Reoptimization of anti2
Anti2 was run at the B3LYP/6-31G* level of theory.
Comparison to anit2 at the HF/3-21G level of theory shows that the difference in the central C-C dihedral is negligable, however the dihedrals adjacent to the central C-C single bond vary by 4 degrees, from 114 to 118 degrees. Overall the geometry does not change significantly.

g) Frequency analysis of anti2
Ran frequency calculation at the B3LYP/6-31G* level of theory on optimized anti2.
All frequencies real, indicating minimum shown in the figure below
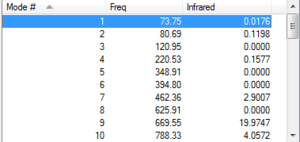
| Task | Energy/ Hartree | Comments |
|---|---|---|
| (i) the sum of electronic and zero-point energies | 234.469215 | E = Eelec + ZPE
where E is potential energy at 0 K, Eelec is electronic energy, ZPE is zero point energy |
| (ii) the sum of electronic and thermal energies | 234.461866 | E = E + Evib + Erot + Etrans where E is energy at 295 K, 1 atm and Evib, Erot,Etrans are vibrational, rotational and translational energies respectively. |
| (iii) the sum of electronic and thermal enthalpies | 234.460922 | H = E + RT
Where H is enthalpy, E is energy , R is gas constant, and T is tempurature |
| (iv) the sum of electronic and thermal free energies | 234.500800 | G = H - TS
Where H is Enthalpy, S is entropy, T is tempurature and G is Free energy |
**COME BACK TO AND DO EXTENSION ****
https://wiki.ch.ic.ac.uk/wiki/index.php?title=Mod:gv_advanced
Optimizing the "Chair" and "Boat" Transition Structures
Aims of section:
Set up a transition structure optimization by
- Computing force constants
- Using redundant coordinate editor and QST2
- Visualizing reaction coordinate
- Running IRC (Intrinsic reaction coordinate)
- Calculating activation energies for Cope rearrangement via 'chair' and 'boat' transition structures.
a) Optimization of allyl fragment and guess transition state
- Allyl fragment drawn on Gaussview and optimized at HF/3-21G level of theory
- 2 optimized fragments pasted onto new molecule group and moved to in guess transition state structure using 'symmetrize' option in Gaussview. Guess chair TS structure shown below
b) Optimization of chair TS
Opt+Freq calculation run to optimize transition state guess structure at the HF/3-21G level.
The optimized structure gives one imaginary frequency with magnitude of 817.87 cm-1 as shown below.

The imaginary frequency corresponds to the cope rearrangement as can be seen in the figure below.
****ADD ANIMATION****
Minimum energy structures have all positive vibrational frequencies and transition states have only one imaginary vibrational frequency.
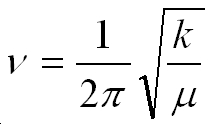
The equation above can be used to calculate the frequency according to the harmonic oscillator approximation, where v is the frequency, k is the force constant and m is the reduced mass.
At a saddle point all the directions coming in are positive, except one ( the one the reaction coordinate will follow), and this will have a negative force constant, which will give a negative square root according to the equation above which gives one imaginary frequency.
c) Optimization of chair TS using frozen coordinate method
The guess chair transition state was then copied into a new molecule group and one set of terminal carbons of the allyl fragments that formed/broke in the cope rearrangement where selected and the bond between them was frozen using the keyword 'opt=modredundant'. This was repeated for the other two terminal carbons. This was then optimized to a minimum at the HF/3-21G level.
The reason for freezing these bonds is to optimize the rest of the molecule without trying t optimize the terminal bonds. These can then be optimized after to find the transition state.
****ADD JMOL****
d) Unfreezing bonds of chair TS and re optimizing to transition state
The above calculation gave a structure similar to that of the TS optimization from part b however the bond distances between the terminal carbons of each allyl fragment is 2.2 angstroms.
Now the same terminal carbons were selected and instead of freezing the bond and optimising to the structure to a minimum, the derivative was taken and the structure was optimised to a transition state as in section b.
A comparison of the structures from part b) and part d) are compared in the table below. Overall they are very similar.
| TS structure | Distance between terminal C's of allyl groups | allyl bond angle | alyll bond length |
|---|---|---|---|
| Optimised without freezing terminal C bonds | 2.02103 | 120.48441 | 1.38922 |
| Optimised with freezing terminal C bonds | 2.02071 | 120.52865 | 1.38930 |
e) Optimization of boat transition state using QST2 method
The QST2 method allows two structures to be inputted, (reactants and products) and an interpolation between them is run to try and find a transition state on the reaction pathway between them.
The structure below shows the output of the qst2 transition state optimization. Clearly this is not the boat transition state and so the calculation has failed. This is due to the interpolation only taking into accound translation and not rotation.
***ASK ABOUT THIS DIDN'T FAIL?****
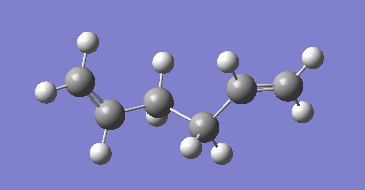
The reactant and product structures were adjusted to look more like the boat structure. The cetral dihedral was adjusted to 0 degrees and the central bond angles were adjusted to 100 degrees. The new input structures for the qst2 are shown below.
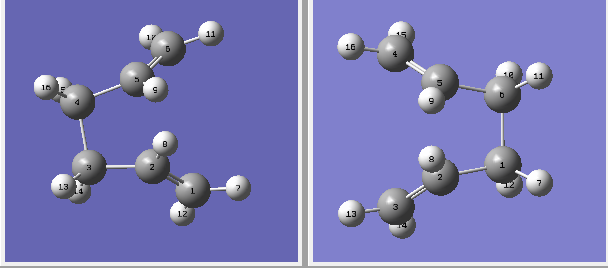
This time the interpolation gives the correct boat structure transition state seen below.
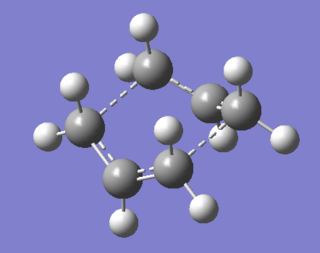
This structure has one imaginary frequency, shown below. This corresponds to the formation/breaking of the terminal C-C bonds.
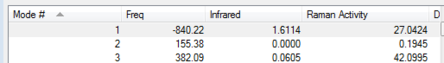
**COME BACK TO AND DO EXTENSION **** - QST3
f) Intrinsic Reaction Coordinate
It is hard to tell which conformers of 1,5-hexadiene they correspond to form appendix 1
Gaussian has a method called intrinsic reaction coordinate (IRC) which follows the steepest gradient of the minimum energy path to a local minimum.
A IRC was run with 50 steps from the chair transition state however this was not sufficient to reach a minimum. The IRC was only run in the forward direction due to the energy pathway being symmetrical. The animation of the IRC and the energy level diagram is shown below.
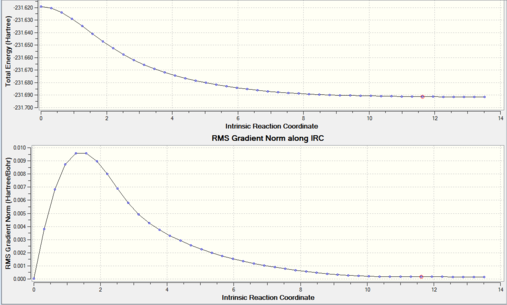
The IRC did not use all 50 steps (only 44) to complete, however the RMS gradient path does not quite reach 0 and so the final structure is not the minimum. There is no point in adding more steps as not all of them have been used. Instead, since the structure was very close to a minimum, the output structure was optimized to a minimum using the HF/3-21G method. The optimization energy diagram and structure are shown below.
The optimized diene had energy -231.69166702 Hartree which corresponds to the gauche2 structure from appendix 1 .

g) Calculation of Activation Energies
| Structure | HF/3-21G | B3LYP/6-31G* | ||||
|---|---|---|---|---|---|---|
| Eelec | Eelec + ZPE | Eelec + Evib + Erot + Etrans | Eelec | Eelec + ZPE | Eelec + Evib + Erot + Etrans | |
| at 0 K | at 298 K, 1 atm | at 0 K | at 298 K, 1 atm | |||
| Chair TS | -231.61932238 | -234.55698264 | ||||
| Boat TS | -231.60280223 | -234.54309307 | ||||
| Reactant (anti2) | -231.69253520 | -234.61171166 | ||||
| HF/3-21G | B3LYP/6-31G* | |||
|---|---|---|---|---|
| at 0 K | at 298.15 K | at 0 K | at 298.15 K | |
| ΔE (Chair) | ||||
| ΔE (Boat) | ||||
The Diels Alder Cycloaddition
Aims of section
- Characterize transition structures
- Look at the shape of some of the molecular orbitals.
In this section the level of theory used for calculations was the semi-empirical AM1 (Austin Model 1) method. This method is suitable to use because it is much cheaper than other methods like B3LYP or Hartree Fock which is purely theoretical. This is due to AM1 being heavily parameterized by using pre calculated empirical data given in the gaussian software. Since the Diels Alder reaction is a simple reaction with small molecules and only Carbon and Hydrogen atoms, the AM1 method gives reasonably accurate results.
Exercise
i) Optimization of butadiene
Butadiene was optimized to a minimum. The HOMO and LUMO are shown below.
ii) Optimization of transition state
Ethylene was also optimized and the two structures where placed near each other and a transition state optimization was run. At first this calculation failed due to the moleucles being to far apart (2.2 angstoms). They were placed closer together and the optimization was rerun. This time a transition state was found. The imaginary freuqency is shown below to prove that it is a transition state.
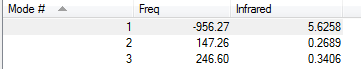
To check that the transition state corresponds to the correct reactants and product an IRC was run. An animation of the IRC and energy diagram are shown below.
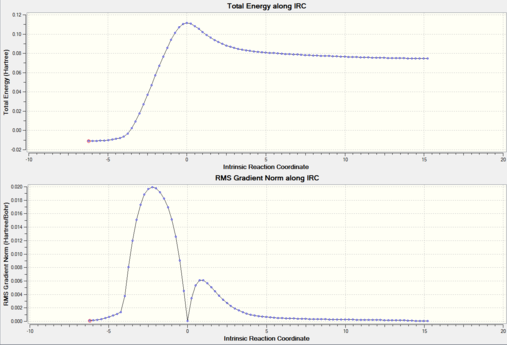
**ADD JMOL ANIMATION****
The HOMO of the transition state can be seen below.
**ADD JMOL HOMO****
iii) Studying regioselectivity of the Diels Alder Reaction
First maleic anhydride and hexadiene were both optimized individually and the optimized structures were put near to each other to make a guess structure. This was then optimized to the endo and exo minimums.
These minimums were then adjusted slightly by lengthening the bond formed in the reaction and the structure was optimized to a transition state. Both structures have one negative frequency and the IRC running in both directions goes to the desired reactants and products. The structures of both transition states and the IRCs are shown below.
****ADD JMOL NORMAL AND VIBRATION FOR EXO AND ENDO TRANSITION STATES***
| Structure | Imaginary frequency Vibration | Imaginary Frequency | Energy/ Hartree | new C -C bond length/ Angstroms | ||
|---|---|---|---|---|---|---|
| Exo | Product | JMOL | n/a | na/ | -0.15990938 | 1.53615 |
| Transition state | JMOL | JMOL | -812.23 | -0.05041982 | 2.17020 | |
| Endo | Product | JMOL | n/a | na/ | -0.16017078 | 1.53577 |
| Transition state | JMOL | JMOL | -806.36 | -0.05150475 | 2.16237 | |
From the data in the table above it can be seen that the endo transition state is more stable. The HOMO of the endo and exo transition states are shwn below. This can be used to explain why the endo transition state is more stable. It is due to secondary orbital overlap. ****
**ADD JMOL HOMO****
Interestingly, although the endo transition state structure is lower in energy than the exo conformer, the endo product and exo product energies are the reverse. The structures of the endo and exo products are shown below. The corresponding energies are xxxxxx and xxxxx hartree respectively. This shows that the endo is the kinetic product so is almost always formed (often referred to as the endo rule), however the endo product is the thermodynamic product as it is the most stable product.
***INSERT ENDO AND EXO PRODUCT JMOLS***
Discussion
cis butadiene
ethylene+cis butadiene transition structure
cyclohexa-1,3-diene reaction with maleic anhydride
Further discussion
- ↑ IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). XML on-line corrected version: http://goldbook.iupac.org (2006-) created by M. Nic, J. Jirat, B. Kosata; updates compiled by A. Jenkins. ISBN 0-9678550-9-8. doi:10.1351/goldbook.
- ↑ IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). XML on-line corrected version: http://goldbook.iupac.org (2006-) created by M. Nic, J. Jirat, B. Kosata; updates compiled by A. Jenkins. ISBN 0-9678550-9-8. doi:10.1351/goldbook.
- ↑ IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). XML on-line corrected version: http://goldbook.iupac.org (2006-) created by M. Nic, J. Jirat, B. Kosata; updates compiled by A. Jenkins. ISBN 0-9678550-9-8. doi:10.1351/goldbook.
- ↑ http://www.chemtube3d.com/DAWoodward-Hoffman.html
- ↑ https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:Pic1.jpg
- ↑ https://bb.imperial.ac.uk/bbcswebdav/pid-523618-dt-content-rid-2176192_1/courses/DSS-CH2_OC-14_15/Sigmatropic.pdf
- ↑ 7.0 7.1 7.2 7.3 https://wiki.ch.ic.ac.uk/wiki/index.php?title=Mod:phys3_appendix1
