Rep:Mod:HarajukuBarbie3
Module 1
The basic techniques of molecular mechanics and semi-empirical molecular orbital methods for structural and spectroscopic evaluations
Modelling using Molecular Mechanics
This exercise aims to investigate using molecular modelling the various aspects of organic reactions such as regio-selectivity, stereo-selectivity and reactivity. The modelling can provide visual as well as numerical evidence to support and in fact suggest possible outcomes of organic reactions. Both molecular mechanic methods as well as molecular orbital methods were used. Having utilised the methods for known molecules an individual and unique investigation was carried out with the above tools.
Part 1: The Hydrogenation of Cyclopentadiene Dimer
Dimerisation of Cyclopentadiene
Cyclopentadiene can dimerise to form both the exo and the endo dimer, however at room temperature it rapidly dimerises to form the endo dimer exclusively.
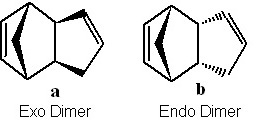
The dimerisation of cyclopentadiene is an example of a Diels-Alder cycloaddition. The relative low energy of the transition state is what enables the reaction to proceed as the delocalised electrons lend aromatic character to the molecule. The reaction, as aforementioned, can proceed with the two cyclopentadiene rings staggered and directly overlapping allowing for the endo product. Or with the rings eclipsed not directly overlapping which allows for the exo product to be formed.
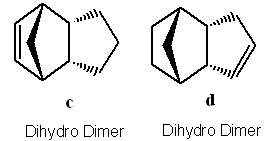
If the preferred dimer is hydrogenated the 2 dihydro derivatives shown are formed. If the hydrogenation occurs over a prolonged period of time then the tetrahydro derivative is formed eventually[1]. The molecules were investigated using the molecular 3D modelling method mentioned in order to optimise their geometries.
| Energies (kcal/mol) | Molecule a | Molecule b |
|---|---|---|
| Stretch | 1.2832 | 1.2616 |
| Bend | 20.5945 | 20.8507 |
| Stretch-Bend | -0.8388 | -0.8354 |
| Torsion | 7.6562 | 9.5055 |
| Non-1,4 VDW | -1.4303 | -1.5164 |
| 1,4 VDW | 4.2388 | 4.3002 |
| Dipole/Dipole | 0.3774 | 0.4451 |
| Total Energy | 31.8809 | 34.0113 |
| Energies (kcal/mol) | Molecule c | Molecule d |
|---|---|---|
| Stretch | 1.2304 | 1.2923 |
| Bend | 18.7590 | 20.5870 |
| Stretch-Bend | -0.7520 | -0.8413 |
| Torsion | 12.7347 | 7.6715 |
| Non-1,4 VDW | -1.3130 | -1.4358 |
| 1,4 VDW | 6.0511 | 4.2320 |
| Dipole/Dipole | 0.1632 | 0.3778 |
| Total Energy | 36.8734 | 31.8834 |
Tables showing the energies of the 2 pairs of dimers and hydrogenated dimers.
The Exo and Endo Products
If the energy values that were obtained using the MM2 forcefield are considered one can see that the energy for the exo product is much lower at 31.8809 kcalmol-1 than the energy for the endo product at 34.0113 kcalmol-1 . The exo dimer's relatively low energy means that the exo dimer can be said to be more stable hence being termed the thermodynamic product. The endo dimer is less thermodynamically stable but as it is produced much quicker is called the kinetic product. In this particular reaction the kinetic product is the one which dominates. This can be supported by the idea of equilibrium, cyclopentadiene dimerises very quickly at room temperature, the Diels-Alder reaction is irreversible meaning the cyclodimerisation is kinetically controlled. This is because the pathway for the reaction proceeds via the most stable transition state that has the lowest activation energy. In this way the reverse reaction does not occur to produce the thermodynamic product.
The Hydrogentaed Products
Molecular Mechanics allows us to predict the more favourable double bond to hydrogenate within the molecules a and b this is by comparing the relative energies of the hydrogenated compounds formed. Molecule dwas found to be significantly more stable having an energy of 31.8834 kcalmol-1 in comparison to the hydrogenated molecule c with an energy of 36.8734 kcalmol-1. Looking at the tables one can see for the hydrogenated species c there is a large bend contribution which is from the stretching and bending of the molecule. Although the energies are acquired at a particular frozen frame in time one can still observe the energy contributions from such effects. For molecule c the is increased strain and therefore decreased stability making d more stable.
Part 2: Stereochemistry of Nucleophilic Additions to Pyridinium Ring (NAD+ analogue)
ChemBio 3D was utilised in order to investigate the nature of two proline derivitives and there conformational behaviour having undergone some form of nucleophilic attack. For both molecules investigated different starting conformations were initially manually inputted in order to allow the overal energy minimum to be found. By altering the molecule into different conformations the overall energy minima could be realised. This was carried out using the MMFF94 force field.
The reaction of N-methyl pyridoxazepinone which is active optically with a Grignard reagent, specifically methylmagnesium iodide was investigated initially. A suggested mechanism by Shultz et al was investigated. The pyridinium ring undergoes alkylation at C-4 position by the grignard reagent in a regioselective and stereoselective way. The Grignard reagent coordinates to the oxygen and the mechanism can be seen in the diagram.
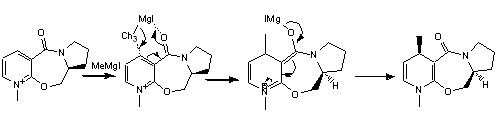
The regio and stereo selectivity can be thought to be governed by the nature of the oxygen present in the amide bond. The concerted reaction allows for the initial alkylation then the second step is pushing back of electrons by the enolate which is now bonded to the magnesium iodide to form the alkylated pyridine ring.
When modelling the various conformers of N-methyl pyridoxazepinone molecule 5 particular attention was given to the positioning of the amide C=O in comparison to the planar (due to aromaticity) pyridine ring. When the geometry of the molecule was optimised in relation to the dihedral angle between the C=O and the carbon being nucleophilically attacked the values collated. It was observed that a dihedral angle of 11.9976o was the one which provided the lowest energy.
Showing that nucleophilic attack occurs when the the carbonyl is orientated above the aromatic pyridine ring. Magnesium is a hard electropositive atom comparitively oxygen is a hard electronegative atom it is therefore favourable for the Grignard to coordinate in the manner shown and deliver the methyl group in concerted step as shown. The selectivity of the reaction using methyl magnesium iodide is relatively high and stated to be 19:1, this can be altered by increasing the steric bulk of the group taking the regio-selectivity up to 99:1.
The Grignard reagent was not incorporated into the calculations for 5when carrying out any of the calculations as ChemBio 3D is unable to identify the magnesium metal and incorporate it correctly. When the magnesium metal was incorporated in to the calculations an error message was produced by the programme. Another difficulty would be with the programme recognising that magnesium was chelated to the the carbonyl oxygen. Gaussian or Mopac would be better suitted models at taking into consideration the idea of partial bonds if one wanted a more accurate system.
If aniline, PhNH2, is nucleophilically added to the N-methyl quinolinium salt a similar sort of selectivity can be observed. In this reaction the steric effects play a much larger role in comparison to the electronic effects. The salt, molecule 7's geometry was again optimised using the same force field for several conformations in order to find the minimum energy conformation to carry out the investigation.
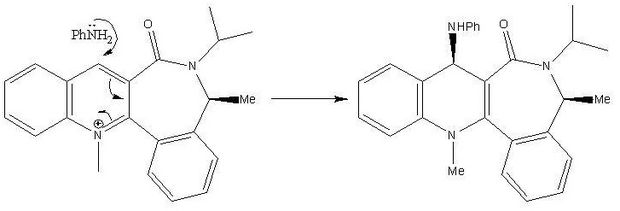
The salts lowest energy conformation was found to contain the carbonyl at -20.3310O below the planar ring. It was found that the most favourable manner of attack by the amine was on the opposite face to the C=OCite error: Closing </ref> missing for <ref> tag. However the C-Cl values were accordance with the literature values of 600-800 cm-1[1]. The observed frequencies were much larger which suggest that the stability of the bond was calculated as being much higher and the bond being much stronger. The overestimation by the model could be due to the fact that a smaller basis set was used to optimise the geometry and obtain the data. Generally the larger the basis set the more accurate a calculation, however this does not always hold true. To further investigate several batches of data could have been obtained using different basis sets to see the imapct it would have on the models accuracy at predicting the strengths of the different bonds.The sysn stretch was noted as being overall more stable than the anti due to its larger frequency.
The experiment was however furthered by the addition of substituents to the anti-alkene and this was done once again to observe specifically the C=C and the C-Cl bonds. The results are shown in the table below:
| Derivative | Syn-alkene stretch | Anti-alkene stretch | C-Cl stretch |
|---|---|---|---|
| Hydroxy substituent | 1758 | 1753 | 765 |
| Cyano substituent | 1757 | 1706 | 766 |
| Silyl substituent | 1756 | 1690 | 764 |
The results show that the C-Cl stretch and as a result the bond was not altered dramatically and seemed to maintain its stability. However the C=C was affected by the substituents as the values altered slightly form those observed earlier. One can see that the Hydroxyl had a stabilising effect on the C=C which is depicted by the increase in frequency this could be due to its ability, more specifically oxygen's ability to donate lone pairs and for a resonance structure. The cyano substituent was the converse as it had a destabilising effect on the double bond due to its inductiive electron withdrawing effect. Finally the silyl substituent also weakened the double bond this could be because silicon has low lying d-orbitals which are able to accept electron density from the electron rich double bond.
Part 3: Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
The molecules below shown in equilibrium are the intermediates in the synthesis of Taxol which is prominent drug used in the treatment of ovarian cancer [2], where the carbonyl can either be upwards facing 9 or downwards 10[3].
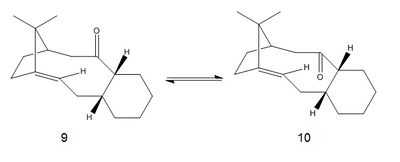
The intermediates 9 and 10 were analysed using ChemBio 3D and both their conformational energies were minimised using the MM2 force field. The intermediates 9 and 10 are cases of atropisomerism as they isomerise to give a different isomer. They are forms of stereoisomers which occur as a result of hindered rotation about single bonds, if the innate barrier to rotation is very high it allows for the two separate conformations to be isolated. Generally the more sterically hindered the molecule the less likely free rotation can occur. The minimum energies were calculated using both the MM2 and the MMFF94 methods with differing force fields and the results were tabulated below:
| Molecule | MM2 Total Energy (kcalmol-1) | MMFF94 Total Energy (kcalmol-1) |
|---|---|---|
| 9 | 50.6088 | 67.4769 |
| 10 | 44.2879 | 60.5642 |
| Molecule 9 | |||
|---|---|---|---|
|
| Molecule 10 | |||
|---|---|---|---|
|
From the energy values present in the table one can see that using both methods the intermediate 10 is much more stable. Different initial starting conformers were inputted into ChemBio 3D in order to ensure that the correct energy minima were obtained. The 4 values were the consistent over an average of 3 initial starting conformers. To obtain the minimum energies for the two isomers the carbonyl groups in the molecules were manually manipulated in ChemBio 3D. It was significantly easier to obtain molecule 10 and the energy converged fairly quickly using both methods. However for molecule 9it was found that the programme took a while to converge and almost everytime converged to the same energy minimum obtained for molecule 10. Showing that although both atropisomers exist isomer 10 is overwhelmingly favoured. The chair conformation was favoured by the cyclohexane ring in 10 whereas the cyclohexane ring in 9 preferred the twist-boat conformation. Because of the change in geometry the cyclohexane ring adopts a higher energy conformation which is why 9 is more destabilised. Other factors which contribute to the destabilisation of isomer 9 is the steric clash between the carbonyl oxygen and the bridging iso-propyl group and also on the same face as the carbonyl are the hydrogen atoms and these can cause transannular strain. In the favoured intermediate the carbonyl is facing down meaning that the steric clash with the iso-propyl group is avoided although transannular strain may still be observed the effect will not be as great.
Due to the closed cage-like structure of the alkenes present in both molecules, their hydrogenation or functionalisation of their relative alkenes are quite slow. These types of alkene's can be described as hyperstable olefins . It was postulated that within pinane cyclic systems the double bonds avoid the junctions in the ring[2]. Maier and Schleyer later observed that the double bond bridge head can be favourable in cyclic medium sized systems. If the hyperstable olefin and its original hydrocarbon's energies are compared this can be rationalised. In hyperstable olefins the olefin strain energies can be observed to be negative making it more stable as it decreases the overall energies of the molecule. By increasing the hybridisation of the carbons from sp2 to sp3 the molecules energy increases. The bond angles decrease and the torsional strain is increased, overall destabilising the molecule with a higher energy.
Structure based Mini-Project using DFT-based Molecular Orbital Methods
Stereoselective Silyl-Directed Stevens [1,2]-Shift of Ammonium Ylides This unique investigation is based on the Stevens rearrangement which involves the rearrangement of ammonium ylides in order to produce C-C bonds which are adjacent to Nitrogen atoms. This reaction was chosen as a starting point to investigate using the methods discussed above due to the fact that the experimental findings show that 2 of the intermediates produced in the reaction were diastereoisomers. This is an example which is frequent in organic chemistry where two isomers are formed and one has to identify and potentially separate the two. Using molecular orbital methods which are DFT based I aim to accomplish the task of distinguishing between two real diastereoisomers. The overall scheme of the reaction is shown
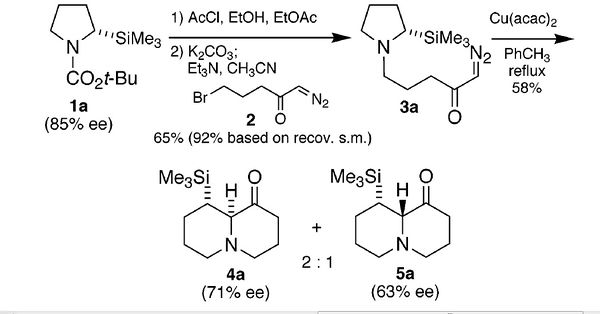
Using 13C NMR as an analytical approach is a good method of attemptin gto distinguish between the two isomers that are made.
Using the GIAO method the predicted 13C NMR were made and compared with those found in the literature adn this was done for both isomers.
| Isomer 4 | Isomer 5 | |||
|---|---|---|---|---|
| Literature | Calculated | Literature | Calculated | |
| 208.0 | 212.8 | 208.0 | 211.8 | |
| 72.7 | 75.8 | 72.7 | 70.1 | |
| 56.5 | 54.3 | 56.4 | 50.2 | |
| 55.3 | 52.7 | 55.3 | 47.8 | |
| 40.4 | 36.9 | 40.5 | 36.8 | |
| 26.3 | 30.6 | 26.3 | 26.1 | |
| 26.2 | 28.6 | 26.2 | 25.7 | |
| 22.7 | 23.8 | 26.2 | 22.4 | |
| -1.2 | -2.0 | -1.2 | -0.9 |
As one can see that literature values were in very strong accordance with molecular orbital predicted values using DFT. This shows that the GIAO method is an extremely powerful tool in allowing one to investigate the structure of molecules such as the ones shown above. The discrepancies in the values show that the DFT method generally underestimated the value of the chemical shifts in ppm, however this could be due to the fact that the method cannot fully account for all the electronic interactions that are occurring. The method also relies on the initial starting molecule before optimisation to be relatively close, and the correct configuration to the original. If for example the bicyclic molecules actually had a lower conformation than initially observed then the calculations would be very far fro the experimental literature. In order to distinguish between the two molecules however further investigations would have to be made as in both the literature and the modelling show that their NMR shifts are very similar showing that another method such as IR or an optical investigation woudl be necessary to further analyse the difference between the two.
Conclusion
Overall the entire exercise was very insightful into being able to see the potential that the methods used have. All investigations carried out initially provided manners in which to explore organic reactions from a slightly different aspect but still obtain data and rationalisations for certain reactions. In some cases the exercise even allowed for predictions of chemical reactivity or regio- and stereo- selectivity. The project allowed the skills acquired to be brought together to give a succinct analysis of a reaction not encountered in the previous section. Overall the exercise allowed for the gaining and exploration into organic reactivity that one never thought possible, bringing this into the modern platform of the internet also shows the scope for ever expanding chemical awareness and knowledge.
References
- A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838. DOI:Error: Bad DOI specified!
- http://www.ch.ic.ac.uk/wiki/index.php/Mod:organic#Stereochemistry_and_Reactivity_of_an_Intermediate_in_the_Synthesis_of_Taxol.
- S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319; DOI:Error: Bad DOI specified!
