Rep:Mod:HSZ15
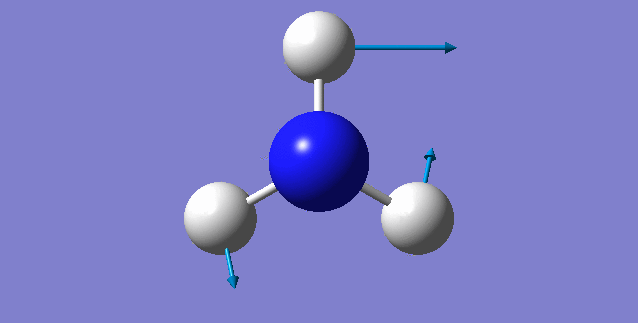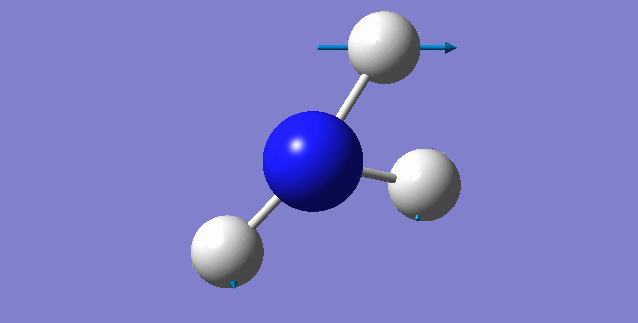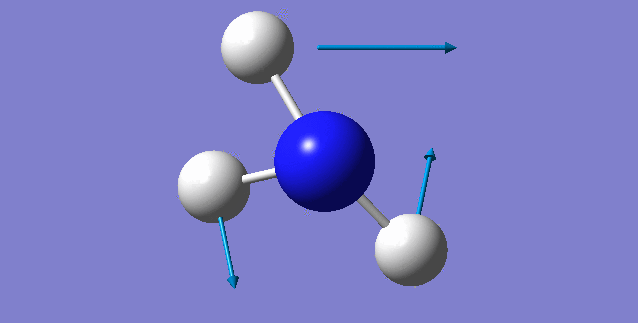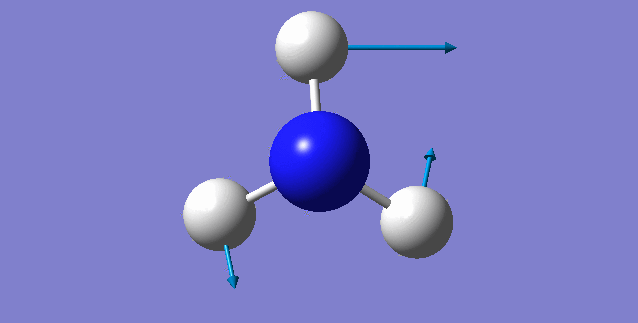
NH3 molecule
Molecule: NH3
Calculation Method: RB3LYP
Basis Set: 6-31G(d.p)
Final energy E(RB3LYP): -56.44397188 a.u.
RMS gradient: 0.00000485 a.u.
Point Group: C3V
N-H bond distance=1.01798 A
H-N-H bond angle=105.741
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
test molecule |
File:HSZ15PHUNT NH3 OPTF POP.LOG
Modes from the 3N-6 rule: 6
Degenerate Modes: Mode 2 and 3 Mode 5 and 6
Bending Vibrations: Mode 1,2 and 3
Bond Stretch Vibraions: Mode 4
Highly Symmetric: Mode 1 and 4
Umbrella Mode: Mode 1
Bands in an experimental spectrum of gaseous ammonia: 4
charge on the N-atom: -1.125
charge on the H-atoms: 0.375
N2 molecule
Molecule: NH2
Calculation Method: RB3LYP
Basis Set: 6-31G(d.p)
Final energy E(RB3LYP): -109.523591111 a.u.
RMS gradient: 0.02473091 a.u.
Point Group: D*H
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
Frequency: 2457.33 No negative frequency
H2 molecule
Molecule: H2
Calculation Method: RB3LYP
Basis Set: 6-31G(d.p)
Final energy E(RB3LYP): -1.17853936 a.u.
RMS gradient: 0.00000017 a.u.
Point Group: D*H
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
Frequency: 4465.68 No negative frequency
The energy for the reaction of haber process
E(NH3)=-56.44397188 a.u.
2*E(NH3)=2*(-56.44397188 a.u.)
E(N2)=-109.523591111 a.u.
E(H2)=-1.17853936 a.u.
3*E(H2)=3*(-1.17853936 a.u.)
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]=2*(-56.44397188 a.u.)-[-109.523591111 a.u.+3*(-1.17853936 a.u.)]
=0.171265431 a.u =0.171265431*2625.5 =449.6573890905 kJ/mol
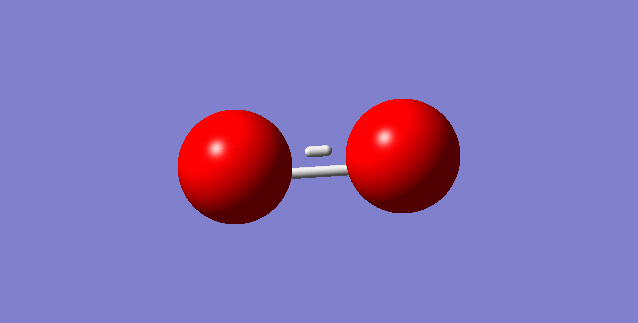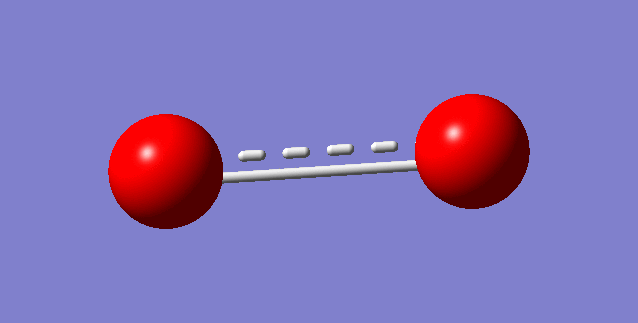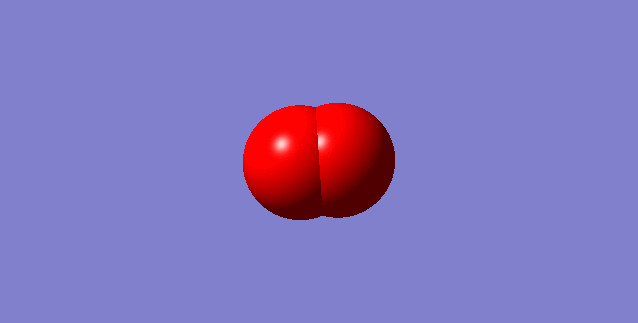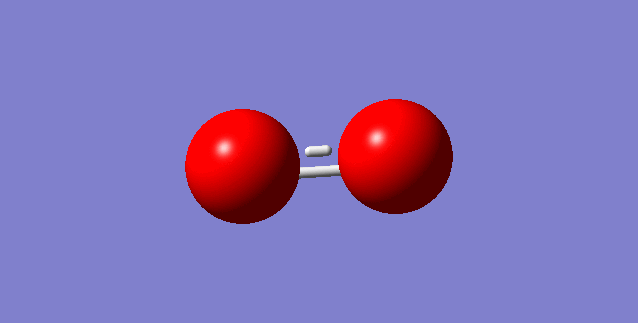
Choice of small molecule O2
Molecule: O2
Calculation Method: RB3LYP
Basis Set: 6-31G(d.p)
Final energy E(RB3LYP): -150.25742434 a.u.
RMS gradient: 0.00007502 a.u.
Point Group: D*H
Bond distance: 1.16160 A
Item Value Threshold Converged? Maximum Force 0.000130 0.000450 YES RMS Force 0.000130 0.000300 YES Maximum Displacement 0.000080 0.001800 YES RMS Displacement 0.000113 0.001200 YES
test molecule |
The frequency of O2: 1642.74
Charge on the O atom: 0.000
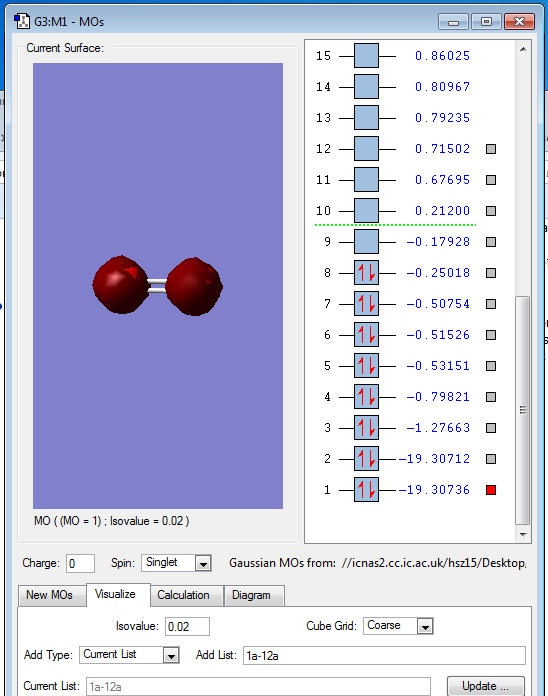 This is the 1s AOs of O2,as 1s orbit has only two electrons.
This is the 1s AOs of O2,as 1s orbit has only two electrons.
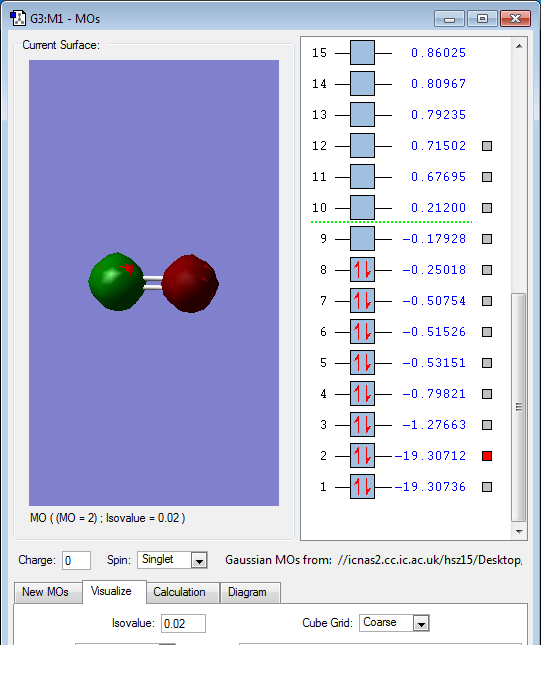 This is the overlap of the p orbital on two Oxygen atoms
This is the overlap of the p orbital on two Oxygen atoms
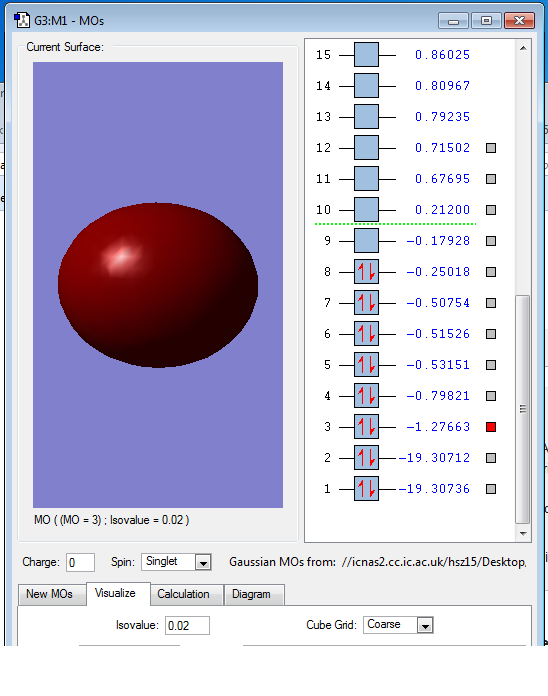 This is the 3s AOs of O2
This is the 3s AOs of O2
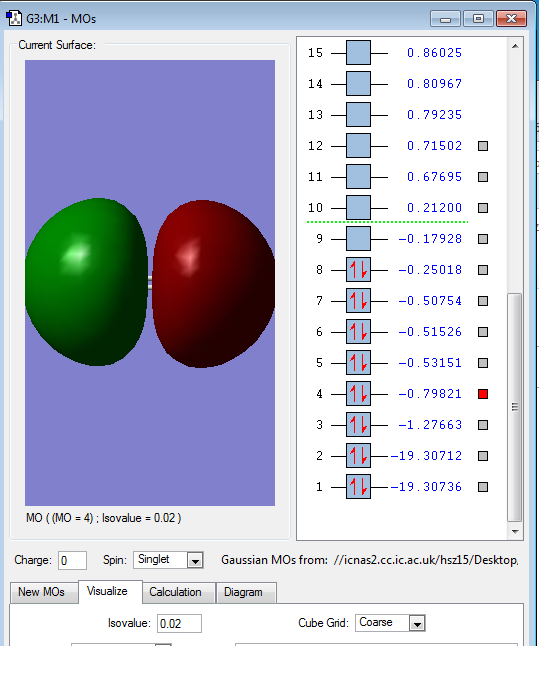 This is the overlap of p orbital on two oxygen atoms
This is the overlap of p orbital on two oxygen atoms
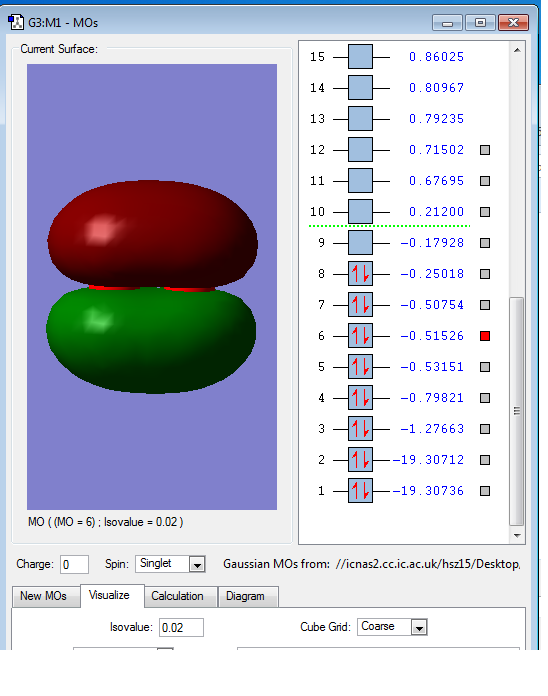 This is also the overlap of p orbital, as it has nearly same energy as 6a orbital
This is also the overlap of p orbital, as it has nearly same energy as 6a orbital