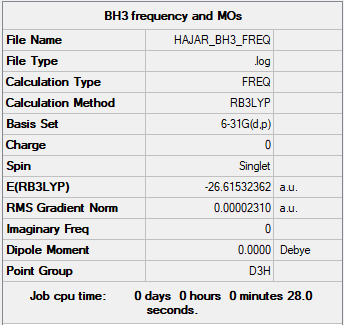
Rep:Mod:HNA4117
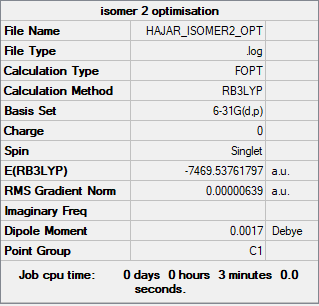
Borane,BH3
Method/Basis set: B3LYP/6-31G
Item Value Threshold Converged?
Maximum Force 0.000046 0.000450 YES
RMS Force 0.000023 0.000300 YES
Maximum Displacement 0.000182 0.001800 YES
RMS Displacement 0.000091 0.001200 YES
Frequency analysis log file: HAJAR_BH3_FREQ.LOG
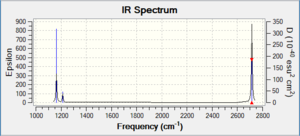
Low frequencies --- -0.4072 -0.1962 -0.0054 25.2514 27.2430 27.2460 Low frequencies --- 1163.1897 1213.3128 1213.3155
BH3 |
Vibrational spectrum for BH3
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1163 | 92 | A2 | yes | out-of-plane bend |
| 1213 | 14 | E | very slight | bend |
| 1213 | 14 | E | very slight | bend |
| 2581 | 0 | A1 | no | symmetric stretch |
| 2714 | 126 | E | yes | asymmetric stretch |
| 2714 | 126 | E | yes | asymmetric stretch |
Although there are 6 vibrations, there are only 3 observed peaks on the IR spectrum. Some of the vibrations have the same wavenumbers as well as intensities indicating that they are degenerate. This gives rise to a single peak. There are two sets of degenerate vibrations. The symmetric stretch does not give rise to a peak because it is IR inactive.
LCAO MOs vs Real MOs
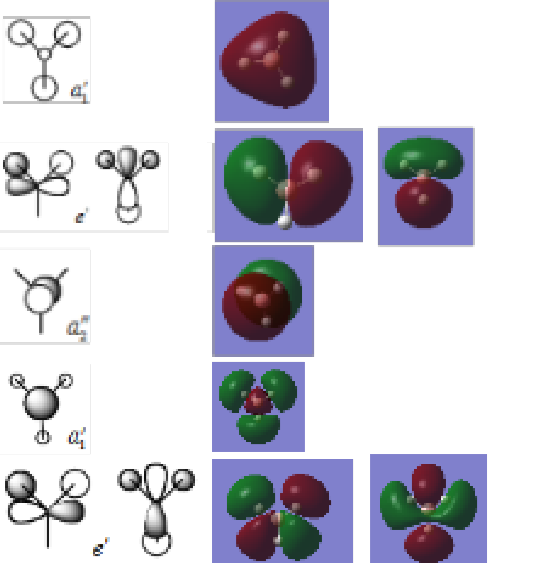
Ng611 (talk) 01:58, 15 May 2019 (BST) Your assignments are correct, although you could have just used the full MO diagram from Trish's lecture and saved yourself the trouble of cropping the orbitals out.
The MOs derived from LCAO are placed next to their respective 'real' MOs. The'LCAO' MOs are taken from Professor Patricia Hunt's lecture course notes http://www.huntresearchgroup.org.uk/teaching/teaching_comp_lab_year2a/Tut_MO_diagram_BH3.pdf
There are no significant differences between the two MOs. The MOs derived from LCAO predicts the 'real' MOs quite well. The extent of diffusion of predicted orbitals vary a little from the real orbitals but generally, the shapes and phases of orbitals are very well predicted.
These predictions from MO theory is a useful method in understanding how particles behave.
Ng611 (talk) 01:59, 15 May 2019 (BST) You mention that the deviate slightly. Could you be more specific?
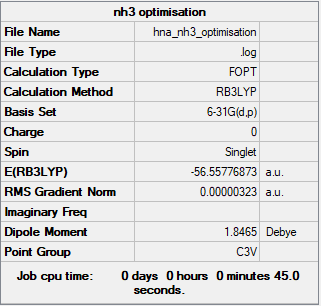
Ammonia,NH3
Method/Basis set: B3LYP/6-31G
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000014 0.001800 YES
RMS Displacement 0.000009 0.001200 YES
Frequency analysis log file: HNA_NH3_FREQ.LOG
Low frequencies --- -8.5646 -8.5588 -0.0041 0.0455 0.1784 26.4183 Low frequencies --- 1089.7603 1694.1865 1694.1865
BH3 |
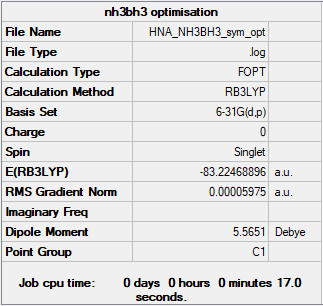
NH3BH3
Method/Basis set: B3LYP/6-31G
Item Value Threshold Converged? Maximum Force 0.000123 0.000450 YES RMS Force 0.000058 0.000300 YES Maximum Displacement 0.000515 0.001800 YES RMS Displacement 0.000296 0.001200 YES
Frequency analysis log file: HNA_NH3BH3_FREQ.LOG
Low frequencies --- -12.7929 0.0011 0.0013 0.0014 19.1979 42.4752 Low frequencies --- 266.1842 632.1568 638.1636
BH3 |
Association energy
E(NH3) = -26.6153 a.u E(BH3) = -56.5578 a.u E(NH3BH3)= -83.2247 a.u ΔE=E(NH3BH3)-[E(NH3)+E(BH3)] = -83.2247 - (-56.5578-26.6153) = -0.0516 a.u * 2625.5 kJ/mol = -135.48 kJ/mol B-N dative bond is quite weak compared to N-N bond which has energy of about 160 kJ/mol or compared to B-B bond of about 293 kJ/mol.
Ng611 (talk) 02:01, 15 May 2019 (BST) Good calculation. You should remember that your calculations are only accurate to about ~1 kJ/mol (and should be reported appropriately). Remember also to cite the sources you obtained your bond energies from!
NI3
Method/Basis set: B3LYP/6-31G
Item Value Threshold Converged?
Maximum Force 0.000061 0.000450 YES
RMS Force 0.000037 0.000300 YES
Maximum Displacement 0.000459 0.001800 YES
RMS Displacement 0.000285 0.001200 YES
My frequency analysis keeps running into error no matter how many people come to help me.
NI3 |
Al2Br2Cl4
Isomer(a) |
Isomer(b) |
Difference in energy between isomer(a) and isomer(b) = 0.005 a.u = -13.13 kJ/mol
From data obtained,isomer (b) with Cl as bridging atoms is more stable than isomer (a) with Br as bridging atoms. Cl is smaller than Br which gives it a better overlap with adjacent Al atom. Al-Cl bond is stronger than Al-Br bond.
Note from editor
I didn't mean to not finish this. I have tried my very best to finish this before 6pm of Friday but I didn't manage to. Persoanlly I find this task difficult and I ran into a lot of error which took a lot of time hence I couldn't finish it, I'm terribly sorry :(
Ng611 (talk) 02:12, 15 May 2019 (BST) I'm really sorry you found it difficult. However, you did excellently in your first section (and managed to pass the lab as a result, which is impressive!). The fact that you were able to do the calculations correctly in section 1 suggests to me that it's well within your abilities to do the work, even if you did struggle.
I'd suggest at some point (when you have some free time), that you go over the second section at a more leisurely pace (and without the pressure of submitting a report) and see how you get on. Otherwise, well done on a good first section!