Rep:Mod:HJW116
NH3
Frequency file: NH3 data file
Ammonia |

|
Energy
 |

|
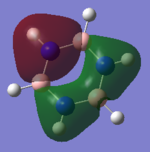
BH3
Frequency file: BH3 data file
BH3 |
Energy
Using GaussView 5.0, a simple BH3 was first drawn, and then optimised. The following figure shows the settings used:
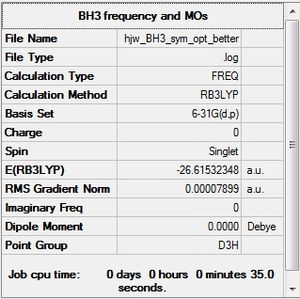 |
To test that the calculation run had come to completion, the data file was observed to see if the data had been converged.
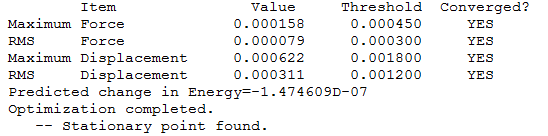
|
Vibrations
With the molecule optimised, the vibrations of the bonds were analysed and a spectrum was taken:

|
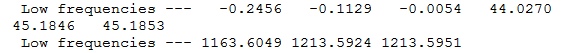
|
Ng611 (talk) 19:07, 21 May 2018 (BST) Remember to round your intensity values to the nearest whole number.
As mode 4 does not result in a net change of dipole moment, it is IR inactive so therefore is not seen in the spectrum. This is why only 5 out of the expected 6 peaks are present. Also, as there are two degenerate sets of vibrations, these apeare as only 1 peak on the IR spectrum.
MOs
With the molecule optimised, the vibrations of the bonds were analysed and a spectrum was taken:

|
| From: http://www.huntresearchgroup.org.uk/teaching/teaching_comp_lab_year2a/Tut_MO_diagram_BH3.pdf |
Ng611 (talk) 19:08, 21 May 2018 (BST) Where are the e' orbitals?
For most of the orbitals the overlap from the real MOs mirror those of the LCAO. However, for the A' anti-bonding orbital the contribution from the H atoms are a lot smaller in the LCAO than that of reality. The accuracy of the bonding orbitals seems to be good, it is only in the higher energy unoccupied orbitals that the MO theory is of lower accuracy.
NH3BH3
Frequency file: NH3BH3 data file
BH3 |
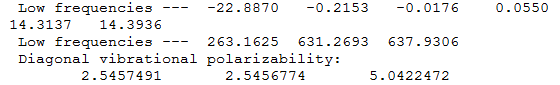
|
Energy
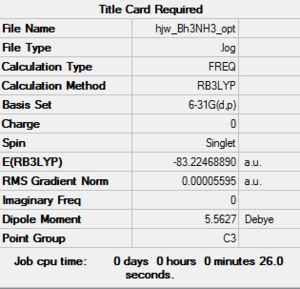 |

|
Energy of associations
| Molecule | E (AU) | E(kJ/mol) |
|---|---|---|
| E(NH3) | -56.55777 | -141,394 |
| E(BH3) | -26.61532 | -66,538 |
| E(NH3BH3) | -83.22469 | -208,062 |
| Difference | -0.0516 | -129 |
Ng611 (talk) 19:09, 21 May 2018 (BST) You got the right energy difference, but you've performed the conversion to Kj/mol incorrectly! There also needs to be a comparison to the literature to put the value you've obtained in context.
BBr3
Link to file: DOI:10042/202389
Frequency file: BBr3 data file
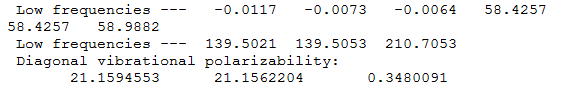
|
BBr3 |
Ng611 (talk) 19:12, 21 May 2018 (BST) Missing an item table here.
PROJECT - Aromatics
Benzene
Frequency file: Benzene data file
Benzene |
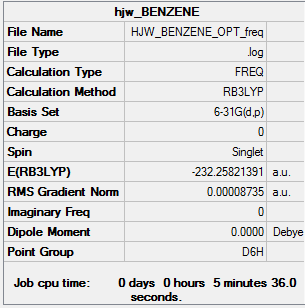
|
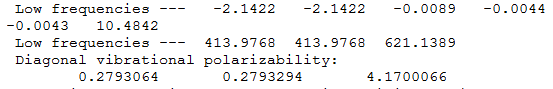
|
Borazine
Frequency file: Borazine data file
Borazine |

|

|
Charge distribution (NBO)
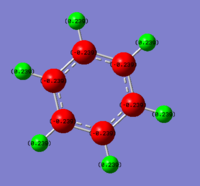 |
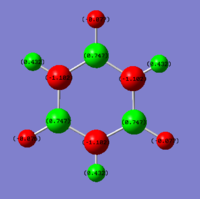 |
| Benzene | Borazine |
Ng611 (talk) 19:13, 21 May 2018 (BST) Remember to use the same colour scale for both molecules.
The charges in Benzene are seen to be evenly distributed, with the C having the partial negative charge (-0.239) and the hydrogen having the partial positive (0.239). The difference in electronegativities of C and H are (2.55-2.20) 0.35, showing that the C has the stronger ability to attract the electrons from the covalent bond.
On the other side; Borazine has a varying charge distribution with the H either being positive (0.432) or negative (-0.077) depending on the hetero-atom it is bonded to. The nitrogen, having the higher electronegativity, causes the H to be partially positive, whereas the 2.04 lower electronegativity of boron leads to the partial charge on the hydrogen being negative and the boron carrying the 0.747 positive.
Ng611 (talk) 19:14, 21 May 2018 (BST) Good discussion of the effects of electronegativity on the overall charge distribution. What do the partial charges sum to, and is there any difference in partial charge for atoms related by symmetry?
MOs
Aromaticity
The first definition of aromatic compounds was from August Wilhelm Hofmann in 1855, who defined them as a "class of benzene-like compounds" many of which had aromas. Later explanations linked aromatics to be molecules with benzene-like structures. The idea of a delocalised cloud of electron density was first thought of by a man called Kekulé who dreamt of a snake eating it's own tail. With further research the idea that the ring was not a tri-ene, but a constantly changing resonance of both structures.
Now we know that, for a molecule to be aromatic, it must follow a set of rules (Hückel's):
1. Must be a planer ring system
2. Must have 4n+2 valence pi electrons
3. Must be fully conjugated
These rules are well established however it can be seen that the planarity of a system can be broken and the molecule can retain aromatic nature. An example of this is benzene at 20K. This is no longer planar (has adopted the chair conformer) yet is still aromatic. It can hold this structure due to the strength of the lattice's inter molecular forces. (Chemistry, A European Journal: Application of AIM Parameters at Ring Critical Points for Estimation of-Electron delocalization in Six-Membered Aromatic and Quasi-Aromatic Rings, Marcin Palusiak*[a] and Tadeusz M. Krygowski[b]) Normally, the aromatic compound must only fill the bonding orbitals in its outer shell, however diphosphatriazolate is 80% aromatic (compared to benzene) yet has two non bonding orbitals filled. This data was gathered experimentally as is non deterinable via calculations.
Molecules with 4n valence electrons are called anti-aromatics. These molecules are actively unstable. This causes most 4n electron systems to adopt a non linear cyclic structure to avoid the de-stabilization.
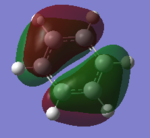
A way of describing armoaticity has been to think of the resonance around the pi system as the pz orbitals overlapping as seen in example A:
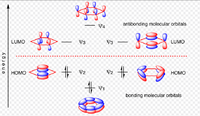 |
This is not a great way of representing an aromatic system as it shows the overlap as a bagel shape, whereas it is seen in the MO theory and calculations, the electron density is more diffused throughout the system. It is therefore more accurate to use MO theory to determine the shape of the orbitals that are responsible for the aromatic nature of these compounds.Also, when people focus only on the pz orbitals, however all the outer orbitals are contributing to the stabilisation of the system, including the sigma, however this isn't well researched.
The delocalised pi ring also causes some odd results in nmr scans. Due to the nature of electric fields within magnetic ones, a counter magnetic field is produced. This causes the outer H atoms to feel extra deshielding and therefore are further shifted when compared to simple alkene adjacent hydrogen. However, in larger aromatics, hydrogen found within the ring feel almost no applied field due and therefore can experience -ve shifts.
Aromatic molecules are characterized by multiple reaction. One being the unique substitution reaction they under-go with electrophiles. This unique reaction allows the swapping of a group around the ring, however after the reaction, the aromatic nature is retained, unlike a cyclo-nucleophile being attacked by an electrophile, which results in the saturation of the C=C bond (or equivalent.) Aromatic can also undergo a multi-molecular interaction known as pi-pi stacking. This occurs when two rings are layered on top of each other. This non-specific interaction is very useful, for example in the Buckycatcher molecular tweezers (two concave buckybowls that are perfectly designed to grab fullerene molecules.) ( A. Sygula, F. R. Fronczek, R. Sygula, P. W. Rabideau and M. M. Olmstead (2007). "A Double Concave Hydrocarbon Buckycatcher". J. Am. Chem. Soc. 129 (13): 3842–3843. doi:10.1021/ja070616p. PMID 17348661.)
Ng611 (talk) 19:19, 21 May 2018 (BST) Some thoughts on how the modern coneptual model of aromaticity has evolved from Huckel's model would improve the section further.
Ng611 (talk) 19:19, 21 May 2018 (BST) A good effort made here. There were a few layout problems (mislabelled JMols and quite a few typos, as well as some missing item tables). However, your calculations were well performed, and your explanations promising. A thorough proofread would have improved this report greatly.