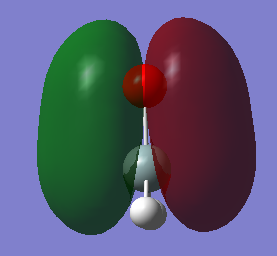Rep:Mod:Gr3GgRLz0k
Ammonia
Introduction
The table below displays key information of the optimized ammonia (NH3) molecule as calculated by GaussView:
| Type | Value |
|---|---|
| Molecule name | Ammonia |
| Calculation method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy (E(RB3LYP)) | -56.55755789au |
| Point group | C3V |
All angles in NH3 is equivalent and at 105.7446o; similarly the bondlengths are all equal at 1.018 angstroms. This is visualized in this figure:
Ammonia molecule |
The table below displays the "item" table as presented in the .log file from GaussView:
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000014 0.001800 YES RMS Displacement 0.000009 0.001200 YES
To view the original .log file, click Media:Nuntapob916_Ammonia_optimised_logfile.log
Vibrations
Ammonia is expected to undergo 6 vibrational modes according to 3N-6 law. The modes, the frequencies and the infrared values of the vibration calculated by GaussViewer are shown in the figure below:

Out of the 6 vibrational modes predicted, there are two degenerate pairs; vibration mode 3 and 2 and vibration mode 5 and 6. Mode 1, 2 and 3 are bending modes whilst Mode 4, 5 and 6 are stretching. Because in mode 4, the bond stretches away from the center atom (Nitrogen) equally and in opposite direction, it is highly symmetrical. Additionally, mode 1's bending characteristics resembles that of an umbrella being opened, it is also referred as the "umbrella" mode.
Due to the degenerate pairs, it is expected that 4 peaks would appear however mode 4, 5 and 6 has such a small change in dipole that the intensity is diminishingly low (1.0594 and 0.5398) only 2 significant peaks (from mode 1, 2 and 3) will appear on an IR spectrum .
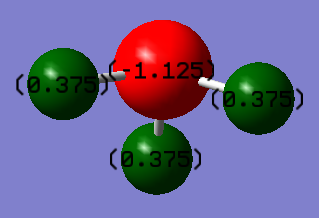
Atomic Charges
In NH3, it is expected that the nitrogen atom would carry a negative charge while the hydrogen atoms will carry a positive charge. This is due to the electronegativity of nitrogen (3.04 pauling units) which withdraws the electron density from the hydrogen atoms (2.20 pauling units). The figure below shows the atomic charges of the atoms in an NH3 molecule:
Synthesis and Reactivity
The modern day synthesis pathway for ammonia is an inorganic synthesis through the Haber-Bosch Process which is simply reacting nitrogen and hydrogen under high pressure with a suitable catalyst.
Listed below are the values optimized and calculated on GaussView of the reactants; nitrogen and hydrogen molecules.
Hydrogen summary
The table below displays key information of the optimized hydrogen molecule (H2) as calculated by GaussView:
| Type | Value |
|---|---|
| Molecule name | Hydrogen |
| calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -1.17856936 a.u |
| Point group | Dinf |
Hydrogen is a diatomic linear molecule with the bond length of 0.7429 angstrom.
Hydrogen molecule |
To prove that the values or optimized, the item list from the original log file is presented below:
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
And an analysis on vibration frequency displays only one mode as expected from a diatomic linear molecule being H-H stretching with a calculated frequency of 4465.58cm-1. This will not show on an IR spectroscopy spectrum due to the high symmetry of the vibration leading to no net change in dipole. The original log file can be accessed through here: Media:NJ916_HYDROGEN_GAS_OPTIMISATION.LOG
Nitrogen summary
The table below displays key information of the optimized nitrogen molecule (N2) as calculated by GaussView:
| Type | Value |
|---|---|
| Molecule name | Nitrogen |
| calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -109.52412868 a.u |
| Point group | Dinf |
Nitrogen is also a linear diatomic molecule but with a bond length of 1.10549 angstroms.
Nitrogen molecule |
The item list from the original .log file is posted below:
Item Value Threshold Converged? Maximum Force 0.000028 0.000450 YES RMS Force 0.000028 0.000300 YES Maximum Displacement 0.000009 0.001800 YES RMS Displacement 0.000012 0.001200 YES
As expected, only one vibrational mode is observed which is the N-N stretching which has a frequency of 2457.41cm-1. This would not appear on the the IR spectroscopy spectrum due to no net change in dipole due to the high symmetry of the vibration. The original .log file could be accessed here: media:NJ916_NITROGEN_GAS_OPTIMISATION.log
Reaction Energy
The reaction of nitrogen and hydrogen in the Haber-Bosch process can be stoichiometrically written as:
N2 + 3H2 → 2NH3
The energy values needed to calculated the reaction energy in the Haber-Bosch process is shown in the table below:
| Molecules | Energy (a.u) |
|---|---|
| NH3 | -56.55755789 |
| 2NH3 | -113.11511578 |
| N2 | -109.52412868 |
| H2 | -1.17856936 |
| 3H2 | -3.53570808 |
The total reaction energy can be calculated by substituting into this equation:
ΔE=E(2NH3)-[E(N2)+E(3H2)]= -0.05527902a.u = -145.14 KJ mol-1
Since in a Haber-Borsch reaction to form ammonia, the reaction is simply forming ammonia from its constituent elements, it will have the same reaction energy as the enthalpy of formation of NH3. The experimental value for enthalpy of formation for ammonia is -46.2 KJ/mol[1]. Since two moles of ammonia is formed in the Haber-Borsch process, the reaction energy is twice of that; -92.4 KJ/mol. The difference in the calculated energy and the experimentally determined energy is significantly large, at 53 KJ/mol which may be due to the over optimization of ammonia as it doesn't take into account the standard conditions at which enthalpy of formation would be carried out at.
H2SiO: The Simplest Silanone
A silanone is the silicon analogue of the organic ketone, with a silicon in place of carbon instead. It consists of an sp2 hybridized silicone center atom bonded to two R groups and a Si=O double bond. The simplest silanone is replacing both R groups with a hydrogen atom which has the chemical formula of H2SiO. The molecule adopts a trigonal planar geometry as shown by the figure below:
H2SiO |
There are two distinct bonding angles in H2SiO, being O-Si-H with an angle of 124.16o, and H-Si-H angle of 111.69o. The predicted Si=O bond makes sense as it is shorter than an Si-O bond in a metasilicic acid at 1.65 angstroms[2].
The key information are displayed below:
| Type | Value |
|---|---|
| Molecule name | H2SiO |
| Calculation method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy (E(RB3LYP)) | -365.90001403a.u |
| Point group | CS |
The item file of the optimisation via GaussView is shown to prove that the molecule has been fully optimised:
Item Value Threshold Converged? Maximum Force 0.000023 0.000450 YES RMS Force 0.000009 0.000300 YES Maximum Displacement 0.000023 0.001800 YES RMS Displacement 0.000017 0.001200 YES
The original .log file could be accessed here: Media:NUNTAPOB916 SILANONE OPT.log
Vibration
Following the 3N-6 law, H2SiO would be expected to go through 6 different vibrational modes, which is what is displayed by GaussView:
Since all 6 vibrational modes are distinct of each other, it would be expected that there would be 6 different peaks at different frequency on an IR spectroscopy spectrum. Although H2SiO has been observed in an IR spectroscopy, the exact value of the IR spectroscopy values and wavenumber are not yet determined. However a matrix IR spectroscopy has been done on a related compound, dimethyl silanone which has methyl group instead of hydrogen. The Si=O bond vibration wavenumber has been observed at 1204.1cm-1[3]. Comparing this to the Si=O bond predicted by GaussianView which gives a value of 1219.23cm-1 (Si=O stretching mode), the values are incredibly similar and the difference may be due to the difference in μ due to methyl groups being heavier than hydrogen and therefore decreasing the frequency in dimethyl silanone when in comparison to H2SiO.
Charge Distribution
The charge distribution of H2SiO is again ruled by the electronegativity of each atoms. Oxygen has a very high electronegativity so it is expected to withdraw away electrons from silicon, highly polarizing the Si=O bond making Si atom delta positive. The hydrogen are slightly more electronegative than the center Si atom therefore further withdrawing away electrons though since the difference in electronegativity is so minute, this is very small. The image below shows the atomic charge distribution of each atom in a H2SiO molecule.
Molecular Orbitals
The central atom, Si, adopts an sp2 hybridized orbital and therefore present a triganal planar geometry. Similarly, the oxygen atom also adopts an sp2 hybridized orbital. The difference is that for oxygen, the 2s and 2p orbitals are involved where as in silicon, being on the third period, it is 3s and 3p instead. Curiously, the 2p orbitals in silicon is not degenerate as it would be in its atomic state even when it is not involved in bond formation at all.
Silicon 2p orbitals
Si=O bonds: The source of instability
The interesting bond in H2SiO however is the Si=O as it is highly polarized and weak. The electronegativity of oxygen is 3.44 and silicon is 1.90. The diference in electronegativity (1.54) is significantly large and therefore displays a highly polarized covalent bond susceptible to electrophilic attacks, even more than that of the carbon analogue; aldehyde/ketone.
The Si=O bond consists of two different bonds, the sigma bond from the overlap of sp2 hybridized orbitals and the pi bond from the overlapping of the p orbitals of silicon and oxygen. The figure below displays the sigma bonding orbital in Si=O:
The two figure below displays the pi bonding orbital and it's respective anti-bonding orbital between the 2p and 3p orbitals orbitals of oxygen and silicon respectively.
Reactivity
In fact, the silanone compounds including H2SiO are so reactive, it could not be isolated and is only observed in argon matrix isolation[4]. As a result it readily reacts with itself to form siloxanes. Siloxanes are polymer compounds with sp3 hybridized silicon atom instead with a Si-O single bond which is more stable and can be easily isolated[4]. The simplest of siloxanes is disiloxanes which has the chemical formula of H6Si2O. A generic structural equation of siloxanes and the structure disiloxane is shown below.
 |
This interactive 3D image is of disiloxane. The calculated Si-O by GaussView is 1.64 angstroms, which is very close to the literature value of 1.621 angstroms[5].
(SiH3)2O |
It is easy to see why disiloxane is thermodynamically favoured as shown by the energy. The optimised energy of disiloxane (-658.88a.u) is much deeper in energy than two separate silanone molecules with an oxygen eliminated (-619.8a.u). The oligomerization is highly enthalpically driven.
The original .log file could be accessed here: Media:NUNTAPOB916 SILOXANE OPTIMISE.log
A possible reaction mechanism for this oligomerization reaction is that the the silanone H2SiO behaves as both a nucleophile and an electrophile. The delta negative oxygen attacks the delta positive silicon atom on another molecule. This propagates on, the possible mechanism is illustrated below:
 |
References
[1] NSHS scince, Standard Enthalpy of Formation (http://nshs-science.net/chemistry/common/pdf/R-standard_enthalpy_of_formation.pdf)
[2] G.V Gibbs, E.P Meager, M.D Newton, D.K Swanson. Structure and Bonding in Crystals, Volume I ed. Tempe Arizona: Department of Chemistry, Arizona State University; 1981. (https://books.google.co.uk/books?id=QGkZya-ZLEAC&printsec=frontcover#v=onepage&q&f=false)
[3] Robert Withnall and Lester Andrews. Matrlx Reactions of Methylsllanes and Oxygen Atoms. Physical Chemistry May 26, 1987; (22901): . http://pubs.acs.org/doi/pdf/10.1021/j100314a006 (accessed 10 March 2017)
[4] Dr. Sakya S. Sen. A Stable Silanone with a Three-Coordinate Silicon Atom: A Century-Long Wait is Over. 2014; (DOI: 10.1002/anie.201404793): . http://onlinelibrary.wiley.com/doi/10.1002/anie.201404793/full
[5] Ronald J. Gillespie and Samuel A. Johnson. Study of Bond Angles and Bond Lengths in Disiloxane and Related Molecules in Terms of the Topology of the Electron Density and Its Laplacian. Inorganic Chemistry 1996; Department of Chemistry, McMaster University, Hamilton, Ontario, L8S 4M1(): 3031-3039. http://pubs.acs.org/doi/pdf/10.1021/ic961381d (accessed 10th March 2017)