Rep:Mod:GTF456
Computational Chemistry Lab
NH3 molecule
Otimised N-H bond length was found to be 1.01798 A and the H-N-H bond angle when optimised was found to be 105.741 degrees.This molecule is linear. The literature bond length for N2 is 1.01A[1] so the calculated value is close to literature.
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
Molecule name: Ammonia
Calculation Method: RB3LYP
Basis set: 6-31G(d.p)
final Energy E(RB3LYP)(au): -56.55776873
Point Group: C3V
Optimised NH3 |
The optimisation NH3 file is linked to here
Vibrations
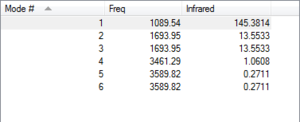
Expected modes from the 3N-6 rule: 6
Degenerate modes are 2 and 3 as well as 5 and 6
Modes 1,2, and 3 are "bending" vibrations whereas modes 4,5 and 6 are "bond stretch" vibrations
Mode 2 is highly symmetric
Mode 1 is known as the "umbrella" mode
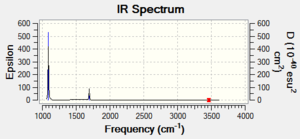
4 bands are expected in experimental spectrum of gaseous ammonia, however mode 4-6 have such a small absorption that the peaks would be indistinguishable above the noise of the spectrum so only 2 peaks would be observed.
Charge
Charge of the Nitrogen in ammonia is -1.125 and the charge of the hydrogens is +0.375. This is as expected as Nitrogen is more electronegative than hydrogen so will have a greater electron density and a negative dipole.
The Haber-Bosch Process
Nitrogen
N-N bond length is 1.10550 A. There is no absorption on the IR spectrum as there is no dipole moment. The literature bond length is 1.45[1] so the calculated value is quite far away from this. This may be due to the gaussian calculation finding the optimum at a subsidiary minimum instead of the actual energy well.
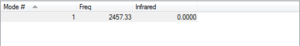
Optimised N2 |
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
Molecule name: Nitrogen
Calculation Method: RB3LYP
Basis set: 6-31G(d.p)
final Energy E(RB3LYP)(au): -109.52412868
Point Group:Dinfh
The optimisation N2 file is linked to here
Hydrogen
H-H bond length is 0.74279 A. There is no absorption on the IR spectrum as there is no dipole moment. This molecule is linear. The literature bond length is 0.74[1] so the calculated value is close to literature.
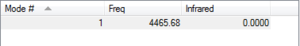
Optimised H2 |
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
Molecule name: Hydrogen
Calculation Method: RB3LYP
Basis set: 6-31G(d.p)
final Energy E(RB3LYP)(au): -1.17853936
Point Group:Dinfh
The optimisation H2 file is linked to here
Reaction
The energy difference between N2 and H2 is: 108.34558932 au Using the equation N2 + 3H2 → 2NH3 it is possible to calculate the energy of the reaction when the energy of the molecules is already known from the optimisation calculations.
E(NH3)=-56.55776873
2*E(NH3)=-113.11553746
E(N2)=-109.52412868
E(H2)= -1.17853936
3*E(H2)= -3.53561808
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]=-0.0557907 au
so -146.48 kJ/mol Therefore the ammonia product is more stable. However the literature value for this reaction is only -91.4 kJ/mol [2]. This big difference in values could be due to errors in minimum energy values. For instance the minimum calculated in gaussian may not be the biggest minimum in energy of the bonds but a subsidiary minimum instead, this may have been for the N2 molecule as the calculated bond length was much further out from the literature values of all the molecules used to calculate the energy of this reaction.
Fluorine Molecule
The F-F bond length is 1.40281 A The bond length is closer to literature values of 1.417 ± 0.001 A [3] but there may be differences due to the optimisation calculation showing a subsidiary minimum instead of the global minimum. There is no absorption on the IR spectrum as there is no dipole moment because fluorine has no overall charge as it is a homonuclear diatomic molecule. The molecule is linear.

Item Value Threshold Converged? Maximum Force 0.000128 0.000450 YES RMS Force 0.000128 0.000300 YES Maximum Displacement 0.000156 0.001800 YES RMS Displacement 0.000221 0.001200 YES
Molecule name: Fluorine
Calculation Method: RB3LYP
Basis set: 6-31G(d.p)
final Energy E(RB3LYP)(au): -199.49825218
Point Group:Dinfh
Optimised F2 |
The optimisation F2 file is linked to here
Molecular Orbitals
The 2p bonding orbital with mixing shows a lower energy than expected of -0.058753 au whereas the LUMO has a higher than expected energy of -0.12679 au showing the mixing of the orbitals. The non bonding orbitals are due to orbitals being so deep in energy that there is no real overlap between them, however there is a slight difference in energy between the two non-bonding orbitals of 0.00007 au due to the phase difference between them - one being out of phase and the more stable one being in phase. The HOMO is an antibonding gerade pi orbital - this can be seen by looking at the image at the center of the molecule. The 2p pi bonding MO however is ungerade and the sigma bonding 2p orbital is gerade. Fluorine has a bond order of one from the molecular orbitals this is seen by the one bond between the atoms.
References
- ↑ 1.0 1.1 1.2 web page: http://www.wiredchemist.com/chemistry/data/bond_energies_lengths.html accessed: 23/03/2017 11:14
- ↑ J.M Madok, resonance, 2002, 69-70
- ↑ web page: http://www.wiredchemist.com/chemistry/data/fluorine-iodine-compounds accessed: 23/03/2017 10:56