Rep:Mod:Fordpage
NH3 optimisation
Here, I exhibit data gained from an optimization of an NH3 molecule.
Molecular data
Bond length: 1.018 Å
H-N-H Bond angle: 105.74°
Final energy: -56.557769 a.u.
Point group: C3V
Charge on N atom: -1.125
Charge on each H atom: 0.375
These charges are to be expected, as N is a strongly electronegative atom, and H much less so, thus N tends to be the focus of most electron density in the molecule. This gives it a large negative charge.
NH3 molecule |
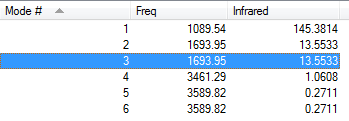
These represent the vibrational modes calclated for an NH3 molecule, along with their predicted intensities on an IR spectrum.
Questions regarding vibrational modes
1. How many modes do you expect from the 3N-6 rule?
The 3N-6 rule would suggest 6 vibrational modes, as observed.
2. Which modes are degenerate (ie have the same energy)?
Modes (2,3) and modes (5,6) are degenerate, based off the numbering of my above table.
3. Which modes are "bending" vibrations and which are "bond stretch" vibrations?
Modes (1,2,3) are primarily bending modes, and (4,5,6) are primarily bond stretching.
4. Which mode is highly symmetric?
Both modes 1 and 4 exhibit strong symmetry.
5. One mode is known as the "umbrella" mode, which one is this?
The umbrella mode is (1), due to its resemblance to a flipping umbrella. This is notable in the phenomenon of nitrogen inversion, whereby a nitrogen centre, such as that in an amine, can easily invert from one chirality to the other, overall meaning that a nitrogen centre is achiral.
6. How many bands would you expect to see in an experimental spectrum of gaseous ammonia?
I would predict that 4 bands are likely to be observed, corresponding to the 4 frequencies of vibrational mode found.
The Haber Bosch process
N2
Data
N-N bond length: 1.105 Å
Final energy: -109.52413 a.u.
No dipole- homonuclear molecule
Point group: D∞h
Only one vibrational mode is present.
H2
Data
H-H bond length: 0.743 Å
Final energy: -1.1785394 a.u.
No dipole- homonuclear molecule
Point group: D∞h
Again, only one mode is present.
Energy calculations
E(NH3)=-56.557769
2*E(NH3)=-113.11554
E(N2)=-109.52413
E(H2)=-1.1785394
3*E(H2)=-3.5356182
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]=-0.055792 a.u.
So overall, the energy change for the process=-146.5 Kj/mol. This indicates an exothermic process, so would suggest that under the conditions of the calculation ammonia is a more stable form than a mixture of Nitrogen and Hydrogen in pure form.
CN- optimisation
Data
C-N bond length: 1.184 Å
Final energy: -92.824532 a.u.
Charge on C atom: -0.754
Charge on N atom: -0.245
Point group: C∞v
CN- Ion |
Only one vibrational mode is present.
Molecular orbitals
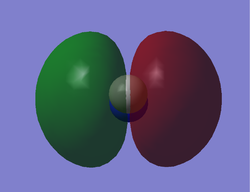
This is the 3σ bonding orbital of the CN- ion, formed from a combination of the 2S AOs of both C and N. As it is clearly spread over the entire molecule, It is likely to be involved in bonding, although its relatively deep energy means it is unlikely to be involved in reactions with other species. One interesting point is the hollowed end near the Carbon, caused by Nitrogen's stronger electronegativity withdrawing electrons from the region.
The first two of these images show the two degenerate 1π bonds present in CN-. Formed from 2 pairs of P orbitals, one each from C and N, these are high in energy, and are strongly involved in bonding between C and N. The third image shows the 5σ MO. This is formed from the third pair of P AOs present, and is the HOMO for the ion. As a nucleophile, the HOMO of CN- will interact with the LUMOs of electrophiles, and the larger bulb of the MO near the Carbon end shows a greater electron density in the region, where an attack is most likely to occur.
This is the LUMO for the CN- ion, the 2π* orbital. It is formed from the one of the P AO pairs in antiphase, and is degenerate with another empty 2π* orbital from the perpendicular P pair. As a nucleophile, the LUMO orbital is not especially important on CN-, as it is both high in energy and protected from attack by the negative ionic charge.
The BH3 dimer
BH3 dimer |
With its electron deficient structure, BH3 is not especially stable as a lone molecule. Instead, it tends to form dimers.
In dimer form, BH3 can complete its octet around both Boron atoms, and avoids unoccupied bonding orbitals. as the above image shows, the MOs are somewhat delocalised over the entire structure, meaning that the dimer is actually quite stable.
One other point of interest are the so-called banana bonds present in the dimer. These are 3 centre-2 electron bonds over the central Hydrogen atoms and the Borons.
Data sources
Calculation data
Calculation method: RB3LYP algorithm
Basis set: 6-31G(d,p)
Item tables
NH3
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
N2
Item Value Threshold Converged? Maximum Force 0.000164 0.000450 YES RMS Force 0.000164 0.000300 YES Maximum Displacement 0.000049 0.001800 YES RMS Displacement 0.000072 0.001200 YES
H2
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
CN-
Item Value Threshold Converged? Maximum Force 0.000105 0.000450 YES RMS Force 0.000105 0.000300 YES Maximum Displacement 0.000047 0.001800 YES RMS Displacement 0.000066 0.001200 YES
BH3
Item Value Threshold Converged? Maximum Force 0.000018 0.000450 YES RMS Force 0.000012 0.000300 YES Maximum Displacement 0.000333 0.001800 YES RMS Displacement 0.000127 0.001200 YES
Links to LOG files
The NH3 LOG file can be found here
The H2 LOG file can be found here
The N2 LOG file can be found here
The CN- LOG file can be found here
The BH3 LOG file can be found here