Rep:Mod:Ferdieinorg19
inorganic comp lab year 2
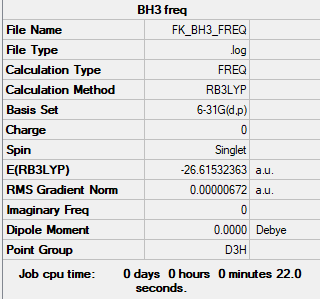
BH 3
B3LYP/6-21G(d,p) level
Item Value Threshold Converged? Maximum Force 0.000013 0.000450 YES RMS Force 0.000007 0.000300 YES Maximum Displacement 0.000053 0.001800 YES RMS Displacement 0.000026 0.001200 YES
Low frequencies --- -7.5477 -1.5512 -0.0054 0.6554 6.9821 7.1523 Low frequencies --- 1162.9679 1213.1635 1213.1662
BH_3 |
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1163 | 93 | A2 | yes | out-of-plane bend |
| 1213 | 14 | E' | very slight | bend |
| 1213 | 14 | E' | very slight | bend |
| 2582 | 0 | A'1 | no | symmetric stretch |
| 2716 | 126 | E' | yes | asymmetric stretch |
| 2716 | 126 | E' | yes | asymmetric stretch |
there are a total of 6 different vibration modes expected however one is symetrical meaning that there is no change in dipole moment and there are 2 bends and 2 stretches which occur at the same frequency meaning that there are only 3 peaks
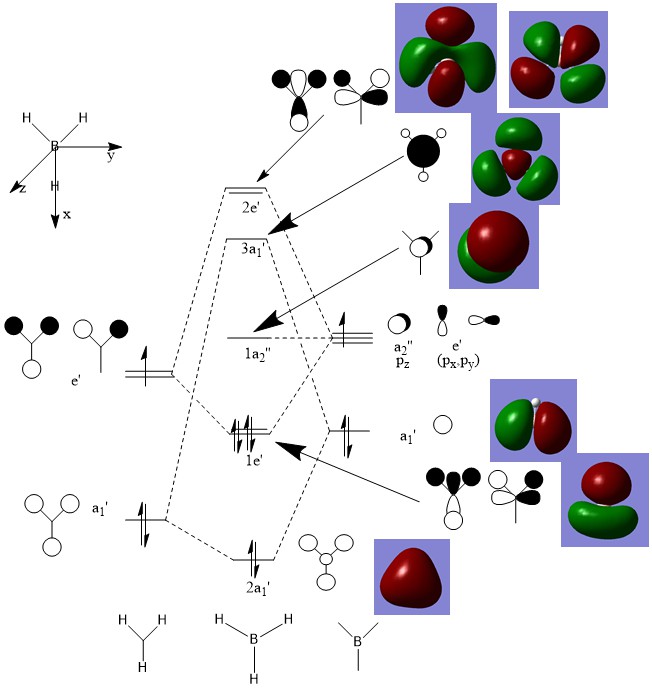
the real MOs and the predicted MOs are in agreement for the bonding orbitals, however for the anti-bonding orbitals they start to deviate from those predicted by the theory. this means that qualitative MO theory is useful for the bonding orbitals but not for the anti-bonding ones and that for existing orbitals it is accurate
Ng611 (talk) 00:31, 22 May 2019 (BST) How exactly is it less accurate? You need to be more specific.
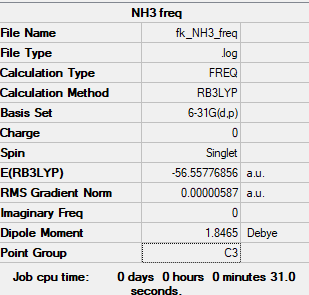
NH3
B3LYP/6-21G(d,p) level
Item Value Threshold Converged? Maximum Force 0.000013 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000039 0.001800 YES RMS Displacement 0.000013 0.001200 YES
Low frequencies --- -8.5646 -8.5588 -0.0047 0.0454 0.1784 26.4183 Low frequencies --- 1089.7603 1694.1865 1694.1865
NH_3 |
spent an 1 hour trying to opt this with Dr Hunt (trying to get C3v sumentry)
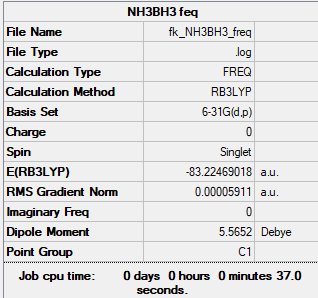
NH3BH3
B3LYP/6-21G(d,p) level
Item Value Threshold Converged? Maximum Force 0.000107 0.000450 YES RMS Force 0.000059 0.000300 YES Maximum Displacement 0.000752 0.001800 YES RMS Displacement 0.000404 0.001200 YES
Low frequencies --- 0.0006 0.0008 0.0011 17.0313 23.8312 44.2509 Low frequencies --- 267.0533 632.2543 638.8808
NH3BH3 |
spent an 1 hour trying to opt this with Dr. Hunt (trying to get C3v sumentry)
Association Energy
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]
E(NH3)= -56.55777 a.u. E(BH3)= -26.61532 a.u. E(NH3BH3)= -83.22469 a.u.
therefore ΔE=-0.05160 a.u. which is -135 kJ/mol this is a senseable number as it is of the order of 100 kJ/mol
therefore this bond is weak when compared to a C-C single bond which has a bond strength of 347kJ/mol
Ng611 (talk) 00:33, 22 May 2019 (BST) You need to cite the source you obtained your C-C bond energy from.
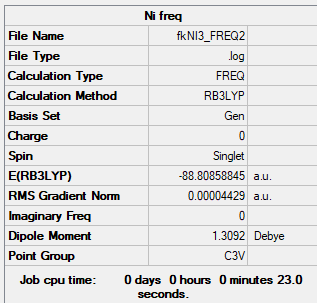
NI3
B3LYP/GEN
Item Value Threshold Converged? Maximum Force 0.000088 0.000450 YES RMS Force 0.000044 0.000300 YES Maximum Displacement 0.000858 0.001800 YES RMS Displacement 0.000481 0.001200 YES
Low frequencies --- -12.3847 -12.3783 -5.6131 -0.0040 0.0194 0.0711 Low frequencies --- 100.9307 100.9314 147.2333
NI3 |
N-I bond length is 2.184Å
Project
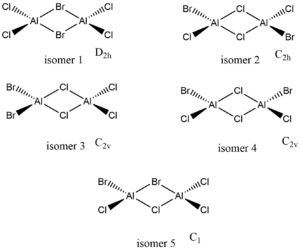
isomers of Al2Cl4Br2
there are 5 different isomers of this compound all with slightly different energies due to the differences in bridging atoms
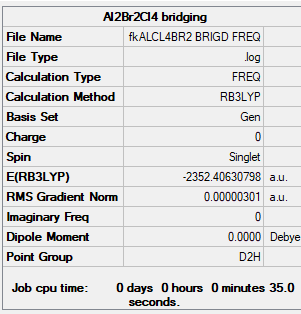
isomer 1 (2 briging Br ions)
B3LYP/GEN level
Item Value Threshold Converged? Maximum Force 0.000007 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.000241 0.001800 YES RMS Displacement 0.000118 0.001200 YES
Low frequencies --- -5.1328 -4.9655 -3.1670 0.0041 0.0044 0.0052 Low frequencies --- 14.8504 63.2858 86.0867
NI3 |
E = -6176243 kJ/mol
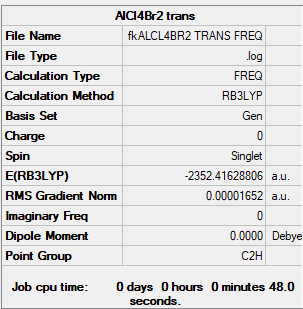
isomer 2 (Br trans with 2 Cl ions bridging)
B3LYP/GEN level
Item Value Threshold Converged? Maximum Force 0.000034 0.000450 YES RMS Force 0.000017 0.000300 YES Maximum Displacement 0.001691 0.001800 YES RMS Displacement 0.000827 0.001200 YES
Low frequencies --- -4.7001 -2.4054 -0.0011 -0.0010 0.0010 0.6321 Low frequencies --- 17.6721 48.9236 72.9633
isomer 2 (trans Br ions) |
E = -6176269 kJ/mol
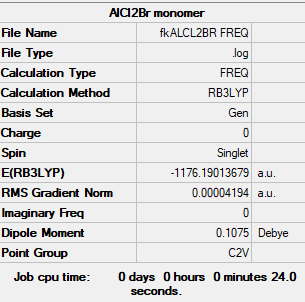
AlBrCl2
B3LYP/GEN level
Item Value Threshold Converged? Maximum Force 0.000081 0.000450 YES RMS Force 0.000042 0.000300 YES Maximum Displacement 0.001588 0.001800 YES RMS Displacement 0.000974 0.001200 YES
Low frequencies --- -0.0033 -0.0015 0.0032 1.3569 3.6367 4.2604 Low frequencies --- 120.5042 133.9178 185.8950
AlBrCl2 monomer |
E = -3088087 kJ/mol
Relative E of isomers
ΔE=E(isomer 1)-E(isomer 2)
therefore ΔE is 26 kJ/mol
this means that Cl ions have a greater stabilising effect on the molecule when they are the bridging ions however this is only a very small amount
Ng611 (talk) 00:38, 22 May 2019 (BST) You need to add a comment about stability, along with a rationalisation.
disociation E of lowest E isomer
dissociation E of isomer=E(isomer)-E(monomers)
therefore dissociation E is -95 kJ/mol
as the dissociation E is negative this means that E is needed to break the bonds meaning that the dimer is more stable
MO of lowest E isomer
Ng611 (talk) 00:42, 22 May 2019 (BST) Excellent LCAO analysis!