Rep:Mod:FM5117
TRANSITION STATES
Introduction
This computational experiment was carried out using Gaussian software. Gaussian is a program for computational chemistry that allows the prediction of multiple properties of molecules and reaction pathways. In this experiment properties such as transitions states, energies, frequencies and vibrations were predicted, to analyse each reaction pathway.
GaussView
GaussView is a graphical interface for Gaussian, which facilitates calculations. For example, it is possible to draw structures on on GaussView, rather than typing in coordinates, and it allows facile manipulation and movement of the molecules prior to optimisation. In this lab, the optimisations were carried out using semi-empirical PM6. More detailed and accurate optimisations were carried out for molecules in exercise 2, using density functional theory -B3LYP- 6-31G optimisation. The latter optimisation technique was much more time consuming due to the higher number of basis set, but ensured better results. When analysing, a balance of time taken and quality output was used when choosing the method.
Nf710 (talk) 10:22, 24 February 2017 (UTC) Higher basis yes but also much higher level of theory, B3LYP is a combined HF and DFT method
Methods and Exercises
Within these options, in this lab, three optimisation methods were used. Method one involved guessing a transition state for the structure, and optimising it directly. Method two involved optimising the guessed transition state to a minimum and freezing the coordinates of the bonds being made before optimising to find the transition state structure. Method three on the other hand involved working backwards form the products to the reactants and was used in exercise 3. In this method, the product molecule was drawn, optimised to a minimum and subsequently bonds being made in the reaction were broken to reveal the starting materials, in the best position for reaction, and method two was applied to find the transition state. Method three was the most reliable for the specific reaction. Deciding whether to use method two or three could be done using Hammond's postulate, Using three if the transition state is thought to resemble the product and two if it resembles the reactants.
Hammond's postulate states that the transition state of a reaction will resemble either reactants or products depending on which one it is closer in energy to.
Pericyclic reactions have a cyclic transition state and form products in a concerted manner. In the exercises below, specifically [4+2] cycloaddition pericyclic reactions are investigated with emphasis on Diels-Alder reactions which are commonly between a diene and a dienophile to form a system containing a cyclohexene.[1]
A cheletropic reaction is also a type of perycliclc reaction, but both bonds made in the concerted addition are made to the same atom of one of the reagents.
Transition State in a potential energy surface:
in a potential energy surface, the transition state is defined as the point with the highest energy. It is a state that is usually short lived and needs to be reached for reactants to form products. Since it is a first order saddle point in a potential energy surface, the vibrational spectrum of the TS contains an imaginary frequency, this is because from the TS, in one direction, an energy maximum is found and in the other, the energy is a minimum. (slope goes up so positive then slope goes down so negative- either side of the TS the direction changes).
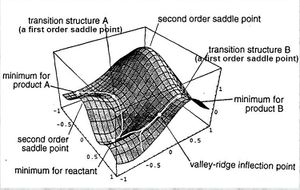
The picture above shows a 3D representation of a reaction pathway.Lit.[2]
Nf710 (talk) 10:33, 24 February 2017 (UTC) You should define this with second derivatives)
Minimum State in potential energy surface:
In a reaction pathway, a minimum state is a dip in the energy. It is effectively a minimum point in the pathway. The lowest minimum point in energy corresponds to the most energetically stable system in the reaction. It is often isolable and long lived. Looking at frequency calculations should show all positive vibrations for this as energy rises either side of the point. The number of imaginary frequencies has to be zero showing the state is observable.
Two imaginary frequencies in a calculation would correspond to a transition state between transition states and the system would last very little in that state.
Running and Intrinsic Reaction Coordinate calculation (IRC) was used in each exercise to confirm the location of the transition state via optimisation. This is because the IRC follows the reaction and finds the minimum energy path. If the path connects the reactants and products of interest, then it is confirmed that the correct transition state has been found.
Diels-Alder reactions:
Diels-Alder reactions can be set up normally or with an inverse electron demand.
A normal Diels-Alder reaction involes a dienophile with electron withdrawing groups and a diene with electron donating groups. This means the LUMO of the dienophile is low in energy and the HOMO of the diene high in energy, thus making them similar in energy and aiding the reaction due to better orbital overlap and stronger interactions. In Inverse Electron demand Diels-Alder, the dienophile contains electron donating groups and the diene electronwithdrawing ones. This means the HOMO of the dieophile is now interacting with the LUMO of the diene, the groups ensure better overlap between these two and stronger bonds, but it is the inverse electron donation compared to normal.
Nf710 (talk) 10:36, 24 February 2017 (UTC) Good understanding of the electron demand of the reaction.
Exercise 1: Reaction of Butadiene with Ethylene
The reaction shown in the scheme below is a [4+2]–cycloaddition, involving a 4 π-electron system (the diene) and a 2 π-electron system (the dienophile)

PM6 optimisation was used to optimise the reactants, products and transition state of the reaction. Method two was used for this because the transition state could be guessed.
Bond Length Analysis

| C-C Bonds | Bond Length (Å) | ||
|---|---|---|---|
| Reactants | Product | TS | |
| C11-14 | 1.327 | 1.540 | 1.382 |
| C1-C14 | / | 1.540 | 2.115 |
| C1-C7 | 1.335 | 1.500 | 1.380 |
| C7-C9 | 1.468 | 1.338 | 1.411 |
| C9-C4 | 1.335 | 1.500 | 1.380 |
| C4-C11 | / | 1.5400 | 2.115 |
Bond length Lit values: C-C single bond is 1.454 Å ,C-C=C double bond is 1.339 Å, Van der Waals radius of C atom is 1.7 Å,.[3]
In this reaction, the bond lengths for C-C bonds correspond to around 1.47 Å, normal lengths for single bonds. Similarly, prior to the reaction, C=C bonds have a length of around 1.33 Å, which again, as seen in literature is an expected double bond length. As the reaction takes place, the bonds can be seen to lengthen, as seen in the Bond length table above. It should be noted that bonds C11-C14 and C9-C4 have a length higher than Van der Waals radii, this is due to the fact these bonds are in the process of being formed. After the transition state, the bonds can be seen to shorten again, as shown by the "product" column in the table above.
(Fv611 (talk) 11:36, 24 February 2017 (UTC) The C11-C14 and C9-C4 bonds are actually shorter than twice the Van der Waals radius of carbon)
MO Analysis
Only atomic orbitals of the same symmetry can interact to make MOs. AOs also have to be close in energy in order to overlap effectively. In this example, ethylene reacts suprafacially with the diene. The MO diagram below was obtained from the optimisations performed.
the HOMO of ethylene and LUMO of the diene combine to form the LUMO of the transition state, since the HOMO of ethylene is lower in energy than the HOMO of the diene. The symmetry labels in the diagram are u for ungrade (antisymmetrical) and g for gerade (symmetrical). The symmetry labels of the MOs arise from those of the AOs since as stated above, they must be of the same symmetry to combine.

MO Visualisation

(Fv611 (talk) 11:36, 24 February 2017 (UTC) The drawing of the TS LUMO is wrong, and you are not using the correct symmetry labels on the TS MOs. Additionally, there should have been a discussion of the orbital overlap integral and its repercussions on allowed mixing)
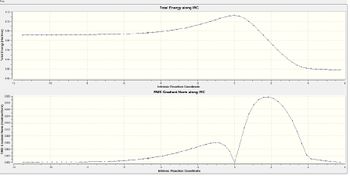
IRC analysis
It could be seen that the optimised structure was the transition state of the reaction due to its imaginary vibration at -948.67 cm-1 . The IRC was used to confirm this, and to show that the correct transition state was reached. The movie below shows the reaction to be concerted, ie both new bonds form simultaneously.
(Fv611 (talk) 11:36, 24 February 2017 (UTC) While you got the right TS, you didn't show the atom movements that give the imaginary frequency as requested.)
The log file for the Transition state can be found here and the log file obtained from the IRC calculation can be found here.
First real vibration was found at 145.09 cm-1. The movie of the vibration can be found below.
From the movie, it can be seen that the vibration for this transition state is asynchronous and corresponds to a wag motion.
Exercise 2: Reaction of Cyclohexadiene and 1,3-Dioxole
The reaction investigated in this exercise involved once again the concerted addition of a dienophile (dioxole) and a diene (cyclohexadiene), however in this example, two products are found to be prevalent: Endo or Exo. This exercise was a great example of how Gaussian energy calculation can be used to determine the main product of a reaction, as well as determining the kinetic product and the thermodynamic product.
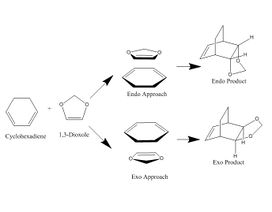
The product formed depended on the approach taken by the dioxole and by the alignment of the orbitals of the reactants.
The calculations were carried out using Method Two, and were optimised using firstly Semi-Empirical PM6, then a more accurate approximation was carried out using B3LYP/ 6-31G(d). Frequency calculations were also run to check that products and reactants had no imaginary vibrations, and that the transitions states both had one imaginary vibration.
MO diagram
The MO diagram below was constructed from the calculations in Gaussian and it can be seen that the reaction involves and Inverse electron demand. This is in accordance to expectation, as the dioxole contained two oxygen atoms, which are electron donating, thus making the structure electron rich. As a result of this, the LUMO of dioxole was too low in energy to interact with the HOMO of the dienophile. the best match in energy was found by overlapping the HOMO of the dienophile with the LUMO of the diene.

(What about the orbitals at the TS? Tam10 (talk) 12:40, 20 February 2017 (UTC))
MO-orbitals:

The IRCs were used to confirm the correct transition state had been found.
(Please include the structures and orbitals for the transition states. Fortunately you've included the log files Tam10 (talk) 12:40, 20 February 2017 (UTC))

The log files for the Transition states with PM6 optimisations can be found here: ENDO , EXO
The log files for the Transition States with 6-31G optimisations ca be found here: ENDO, EXO
The IRC files in the PM6 optimisations can be found here: ENDO, EXO
| Endo product | Exo product |
The movies below taken from the IRC calculations, show that the reactions occur in a concerted manner, as previously thought.


Thermodynamics Section
The reaction energy barrier was found by subtracting the energy of the starting materials from that of the transition states. The reaction energy was found by subtracting the energy of the products from those of the starting materials.
The units were converted from a.u. to kJ/mol.
| PM6-Exo | Pm6-Endo | B3LYP/6-31G(d)-Exo | B3LYP/6-31G(d)-Endo | |
|---|---|---|---|---|
| Reaction Barrier Energy (kJ/mol) | 0.7193870548 | 14.73168162 | 5.657952931 | 3.244068047 |
| Reaction Energy (kJ/mol) | -264.26447163 | -248.18590841 | -217.9664011 | -137.48431797 |
From this data, the Endo and Exo pathways could be compared and the diagram below drawn:

The energy calculations showed the Endo product to be the kinetic product because it had a lower activation energy, but higher product energy. The Exo product was the thermodynamic product: the overall product energy was lower than the Endo, but required a higher activation energy to achieve it.
The lower activation energy of the Endo was due to favorable secondary orbital interactions. Oxygen was sp2 hybridised, and as a result, the p orbital contained lone pairs. In the Endo approach, these orbitals interact with pi bonds on the diene, thus causing a stabilising interaction which stabilises the transition state for the Endo reaction pathway. In the Exo approach, these orbitals are too far away to interact, therefore this stabilising secondary orbital interaction is not observed. [1]
Nf710 (talk) 10:44, 24 February 2017 (UTC) These reaction energies and activation energies are way off, you must have miss calculated them. You have the correct TS for the endo, but the log file you have provided for the exo is a mod redunant calculation. even so I think you have messed up the actually differences in energies
Nf710 (talk) 10:52, 24 February 2017 (UTC) Your arguement for the secondary orbital interaction was good howevr, you still dont have the correct answer for the thermo, because you calculated incorrectly.
Exercise 3: Diels-Alder vs Cheletropic
In this exercise, the cycloaddition between O-Xylylene and sulfur dioxide was investigated. As shown in the reaction scheme below, 3 main products were found: endo and exo Diels-Alder reaction products and a cheletropic reaction product.

(Curly arrows are a bit dodgy. Half-arrows are used for single-electron motion Tam10 (talk) 12:40, 20 February 2017 (UTC))
The optimisations were carried out using Method 3 for the Diels-Alder reactions and method 2 for the cheletropic reaction. All were optimised using the semi-empirical PM6 method.
IRCs
The table below shows the IRC pathways and graphs for each reaction
| Type | IRC movie | IRC Graph | |
|---|---|---|---|
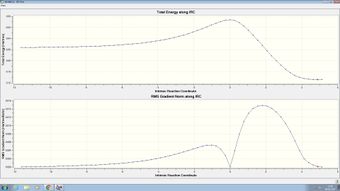
| Endo |  |
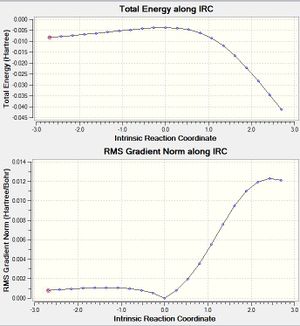 | |
| Exo |  |
 |
(The first IRC graph is incomplete - only 10 steps Tam10 (talk) 12:40, 20 February 2017 (UTC))
The chelatropic file was found to have a minimum at the TS, however the IRC was not found to start at the TS. Several calculations were run and only one was found to have a negative frequency (showing a TS was formed)- but the IRC of reaction did not show a point of zero gradient. Other IRCs seemed to begin from a point that was not a transition state. Both methods 2 and 3 were used to try and obtain this. If time had allowed it would have been beneficial to attempt more calculations, since it is likely a small adjustment in symmetry or bond length could have yielded the correct transition state. However the reaction does appear concerted as expected, and yields the expected product with an energy in the expected range.
Cheletropic:

Literature states that the reaction mechanisms for the Diels-Alder reactions are concerted.[4]
(I don't think this is the same reaction. Looks like they're talking about m-xylene while we're using o-xylylene Tam10 (talk) 12:40, 20 February 2017 (UTC))
From the movies, the reactions would appear to not be concerted. This was very likely due to the oxygen atom of the sulfur dioxide being too close to the xylylene, which explains why the oxygen carbon bond forms just before the oxygen sulfur bond.
The log files for the transition states can be found here: ENDO, EXO, Cheletropic
The files for IRCs can be found here: ENDO, EXO, Cheletropic
Energies
From the calculations above it was possible to obtain the table below and from that the reaction profile diagram was drawn.
| Reaction | Activation Energy barrier (kJ/mol) | Reaction Energy (product energy) (kJ/mol) |
|---|---|---|
| D-A Exo | 4.342577331 | -181.0781233 |
| D-A Endo | 1.278618597 | -180.4427522 |
| Cheletropic | 8.41884354 | -237.4134811 |
(These activation energies are way too low. This might mean whatever you used for the reactant energy was incorrect, but you haven's said what you used Tam10 (talk) 12:40, 20 February 2017 (UTC))

Overall it was found that as with exercise 2, the endo product was kinetic and exo thermodynamic, also due to secondary orbitals stabilisation interactions of the endo transition state. The cheletropic product was found to have a very high energy of activation. This was likely due to electron repulsion in forming a 5-membered ring and steric repulsion between oxygen and sulfurs. However, optimisation of the product shows it to be much lower in energy than endo or exo- showing it to be the most stable product. This could be due to the enthalpic driving force brought about by having two strong S=O intact and not breaking this bond.
Xylylene Instability

The instability of xylylene is due to the ease of formation of delocalised pi systems and benzene rings from it. As with the Diels-Alder reactions shown above, another pathway possible could be the formation of benzocyclobutane, driven thermodynamically by the formation of an aromatic ring. This can be seen by looking at the IRC,s where the 6-membered ring shows dashed lines and the diene part of the molecule shows the possible formation of a four membered ring. However, the reaction is forbidden by Woodward-Hoffman rules, and would therefore require a large energy input to actually occur, for example UV light or high temperatures. However, once the high energy barrier has been surpassed the product is thermodynamically fairly stable and as a result could easily be formed if the SO2 is far away from the xylylene.
Conclusion
In this computational lab, different Diels-Alder reactions were investigated. For exercise 2 and 3, the major products of the reactions were predicted using energies obtained from the calculations, and the reaction mechanisms were found. Furthermore, the IRC showed possible side reactions and reaction path taken.
Methods 2 and 3 were used when optimising these molecules and transition state. Although method 2 was easier to use, it was less reliable due to the need for the transition state to be guessed prior to calculation. Guessing the transition states was possible in these exercises, but might not necessarily be applicable to larger and more complicated systems.
In exercise two, it was found that although the thermodynamic product was exo, the endo product was formed preferentially due to secondary orbital interactions which brought stability to the molecule. In exercise three, the same was found to occur. Further side reactions were also found, for example cheletropic reaction which was found to be the most thermodynamically stable.
Overall this lab showed a computational methods to be in very close agreement to literature data, showing computational predictions carried out using these methods to be accurate and a great tool in predicting and understanding molecular interactions or a specific reaction pathway. Further uses were found in the models' ability to successfully predict major products as well as side products.
The ability to visualise orbitals and molecular orbitals was also a great advantage to aid reaction understanding, by being able to see the orientation, size and overlap of orbitals.
References
- ↑ 1.0 1.1 J. Clayden, N. Greeves and S. Warren, Organic Chemistry, Oxford University Press, 2nd edt.
- ↑ http://images.slideplayer.com/25/7785659/slides/slide_3.jpg.
- ↑ F. H. Allen, O. Kennard, D. G. Watson, L. Brammer, A. G. Orpen and R. Taylor, Journal of the Chemical Society, Perkin Transactions 2, 1987, , S1-S19. .
- ↑ S. Sinha, A. Raj, A. S. Al Shoaibi and S. H. Chung, The Journal of Physical Chemistry A, 2015, 119, 9889-9900.
