Rep:Mod:FITF12
Paclitaxel or its trademark name Taxol is a drug used in cancer chemotherapy. Figure 7 shows an important intermediate, compound C, in the synthesis of Taxol. Compound C, carbonyl group pointing up, can isomerise to an atropisomer where the carbonyl group in the molecule is pointing down, compound D. Furthermore, the alkene functional group in both compounds C and D show uncharacteristic slowed reactivity.
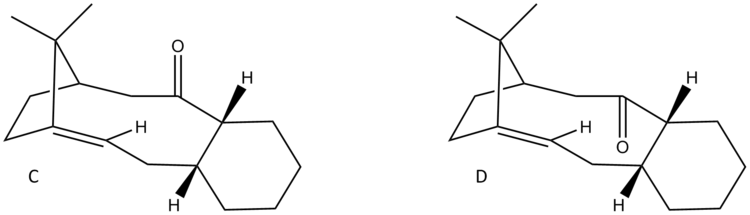
Atropoisomers are stereoisomers that arise due to restricted rotation about single bonds; the steric strain barrier to rotation is too high resulting in separate isomers. It was found (using 3D modelling and geometry optimisation) that both compounds C and D each have a possible of four different conformers; two chairs and two twist boats. Twist boat conformations are higher in energy and therefore are less stable than chair conformations due to greater angle and torsional strain. The more thermodynamically stable conformation (lowest total energy) is the major product and to find this, molecular mechanic calculations were performed on the chair conformations of both C and D. (The energies of the twist boat conformations of both C and D were not regarded as their energies are already high.)
 |
 |
 |
The following data was collected after optimising the geometries (using MMFF94s force field) of the species (twist boat structures had to be modified manually):
| Property | C conformer 1 | C conformer 2 | D conformer 1 | D conformer 2 |
|---|---|---|---|---|
| Total energy (kcal/mol) | 70.54 | 82.82 | 60.56 |
For each compounds C and D, its conformer 1 is the most thermodynamically stable (lowest in energy). Below, they are compared in greater detail to explain why conformer 1 of isomer D is more stable than conformer of isomer C.
| Property | C conformer 1 | D conformer 1 |
|---|---|---|
| Stretch (kcal/mol | 7.71 | 7.59 |
| Bend (kcal/mol | 28.31 | 18.82 |
| Stretch-bend (kcal/mol | -0.07 | -0.14 |
| Torsion (kcal/mol | 0.10 | 0.27 |
| Van der Waals (kcal/mol | 33.23 | 33.23 |
| Electrostatic interactions (kcal/mol | 0.30 | -0.05 |
| Total energy (kcal/mol | 70.54 | 60.56 |
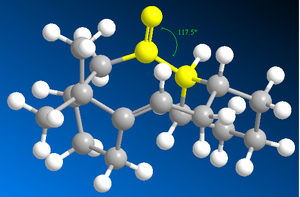
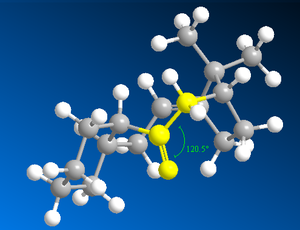
Studying Table 4, it is clear the energy parameter with the largest difference between molecule C and D is the bending term (the deviation from optimal bond angles). The bending term is 9.49 kcal/mol lower for conformer 1 of D than conformer 1 of C. This suggests that the bonding angle for conformer 1 of D is closest to optimal angle thus the overall energy of the molecule is lower since the angle strain is lower (and thus less repulsion from steric clashing). The optimal bond angle for the carbonyl carbon (sp2 hybridised) is 120°. For conformer 1 of D, the bond angel for this carbon is 120.5° (Figure 9) and for conformer 1 of C it is 117.5° (Figure 8).
Hyperstable alkenes are a class of molecules which are less reactive than the more ‘traditional’ alkenes. They have less strain than their corresponding parent hydrocarbon (and therefore negative Olefinic Strain Values – a measure of strain which is calculated by taking the difference between the strain energy of the alkene and its parent hydrocarbon). This reduced reactivity is not because of steric hindrance or a stronger π-bond but because of a cage structured (resulting in unfavourable H-H interactions which make the double bond unreactive) and the greater strain of the parent alkane. To prove this statement, the bending and torsional energies of the parent cycloalkane of isomers C and D were calculated.
The bending term increases by 3.75 kcal/mol (from 28.31 to 32.06 kcal/mol) going from isomer C (conformation 1) to its parent alkane and by 5.83 kcal/mol (from 18.82 to 24.65 kcal/mol) going from isomer D to its parent alkane. Similarly, for the torsional term, the torsional strain increases by 9.35 and 8.42 kcal/mol for isomer C and D respectively.
These increases in the bending term for the parent alkanes can be shown by studying the 3D molecular structures of the parent alkanes. The bond angles at where the alkene was positioned before hydrogenation were measured. The bond angles for isomer C and D were 117.3° and 123.7° respectively. The optimal bond angle for a sp3 hybridised carbon (alkane carbon) is 109.5°, therefore these bond angles are very unfavourable thus causing considerable strain in the molecule.
Furthermore, there are also unfavourable Van der Waals (VDW) interactions between the vicinal hydrogens in the parent alkanes because the structure is ‘cage-like’.
Spectroscopic Simulation using Quantum Mechanics
| Predicted CS | Literature | |
|---|---|---|
| 4.84 (dd,J=7.2,4.7Hz, 1 H) | ||
| 3.40-3.10(m,4H) | ||
| 2.99(dd,J=6.8, 5.2 Hz, 1 H) | ||
| 2.80-1.35 (series of m, 14 H) | ||
| 1.38 (s, 3 H) | ||
| 1.25 (s, 3 H) | ||
| 1.10 (s, 3 H) | ||
| 1.00-0.80 (m, 1 H) |
| Predicted CS | Literature | |
|---|---|---|
| 218.79 | ||
| 144.63 | ||
| 125.33 | ||
| 72.88 | ||
| 56.19 | ||
| 52.52 | ||
| 48.50 | ||
| 46.80 | ||
| 45.76 | ||
| 39.80 | ||
| 38.81 | ||
| 35.85 | ||
| 32.66 | ||
| 28.79 | ||
| 28.29 | ||
| 26.88 | ||
| 25.66 | ||
| 23.86 | ||
| 20.96 | ||
| 18.71 |
