Rep:Mod:FEG17
NH3 molecule
Calculation Method=RB3LYP
Basis Set=6-31G(d,p)
E(RB3LYP)=-56.55776873 a.u.
RMS gradient=0.00000485 a.u
point group=C3V
N-H bond distance =1.01865
H-N-H bond angle =105.749
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986266D-10
Optimization completed.
-- Stationary point found.
NH3 |
From the 3N-6 rule we would expect 6 modes
2 modes #5 and #6 are degenerate
Bending vibrations:#1, #2, and #3
Stretching modes:#4, #5, and #6
The highly symmetrical mode is #4
The umbrella mode is #1
You would probably only see two bands in the IR spectrum of ammonia as the 145.3814 value and the two degenerate 13.5533 values would be easily visible however the other values are very small <1.0754 and therefore would likely not be visible on the spectrum and so only two bands will be seen.
The nitrogen atom has a charge of -1.125 and the hydrogen atoms all have a charge of 0.375.You would expect the hydrogens to be positive and the nitrogen to be negative as the hydrogen are more electropositive and the nitrogen more electronegative.
H2 molecule
Calculation method=RB3LYP
Basis set=6-31G(d,p)
E(RB3LYP)=-1.17853936 a.u.
RMS gradient=0.09719500 a.u.
Point group=D*H
H-H bond distance= 0.74279
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.164080D-13
Optimization completed.
-- Stationary point found.
H2 |
N2 molecule
Calculation method=RB3LYP
Basis set=6-31G(d,p)
E(RB3LYP)=-109.52359111 a.u.
RMS gradient=0.02473091 a.u.
Point group=D*H
N-N bond distance=1.10550
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-3.401074D-13
Optimization completed.
-- Stationary point found.
N2 |
Formation of NH3
E(NH3)=-56.55776873 a.u.
2*E(NH3)=-113.1155375 a.u.
E(N2)=-109.52359111 a.u.
E(H2)=-1.17853936 a.u.
3*E(H2)=-3.53561808 a.u.
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]=-113.1155375--113.0592092=-0.05632831 a.u.
-0.05632831*2625.5=-147.8899779 kJ/mol =-147.89 kJ/mol (2.d.p)
ammonia is more stable as it is an exothermic reaction.
Project Molecule
F2 molecule
Calculation method=RB3LYP
Basis set=6-31G(d,p)
E(RB3LYP)=-199.49825218 a.u.
RMS gradient=0.00007365 a.u.
Point group=D*H
F-F bond distance=1.40281
Both atoms are neutral in f2
Item Value Threshold Converged?
Maximum Force 0.000128 0.000450 YES
RMS Force 0.000128 0.000300 YES
Maximum Displacement 0.000156 0.001800 YES
RMS Displacement 0.000221 0.001200 YES
Predicted change in Energy=-1.995024D-08
Optimization completed.
-- Stationary point found.
F2 |
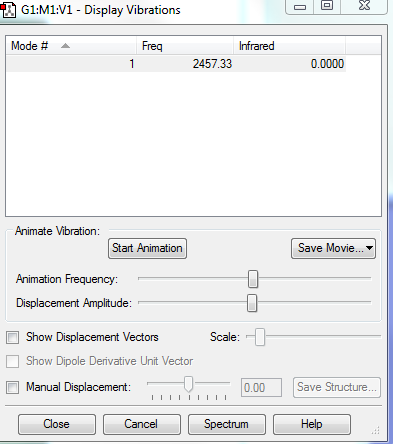
There is only one bond stretching vibration.
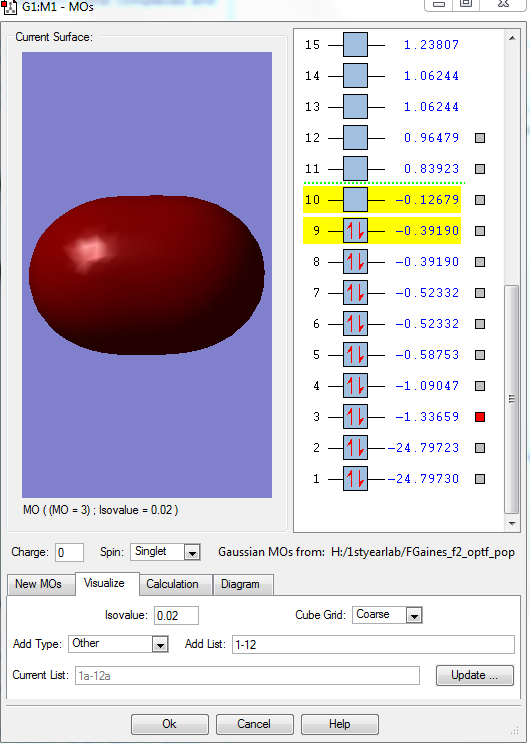
This shows the bonding MO formed when the two 1s AOs of the fluorines combine it is deep in energy at -24.7970 a.u. , occupied, they hardly overlap and are not very involved in chemical bonding.
This shows the bonding MO formed when the two valence 2s AOs of the fluorines combine. It is occupied, and is much higher in energy at -1.33659 a.u., the two AO have overlapped more strongly so only a surface is seen. They are very involved in chemical bonding, making bond stronger.
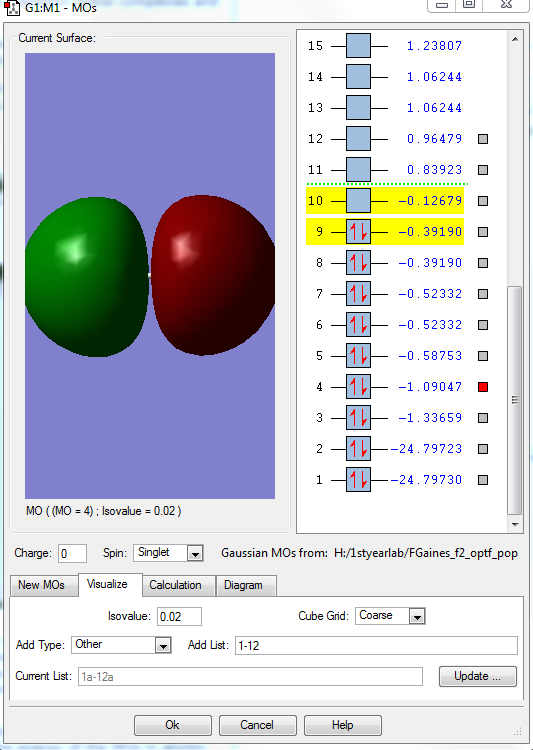
This shows the antibonding MO formed when the two valence 2s AOs of the fluorines combine. It is occupied, and is much higher in energy at -1.09047 a.u. than the 2s bonding orbital , there is a node between the two atoms. They are very involved in chemical bonding, it makes the bond weaker.
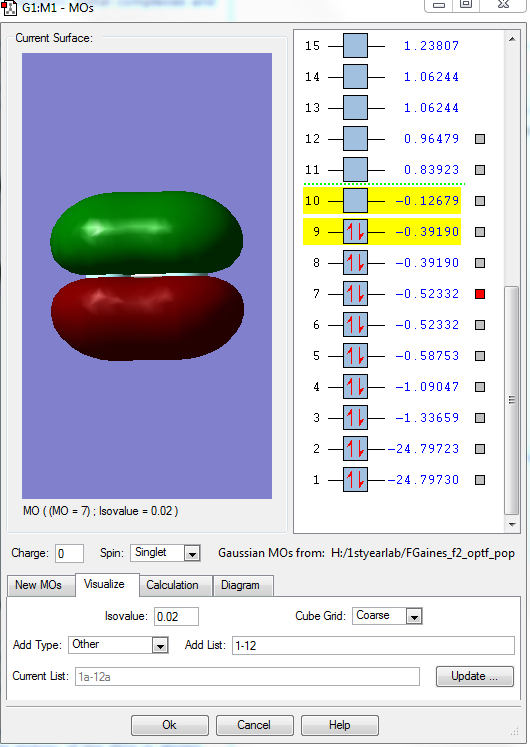
This shows the occupied bonding MO that is formed from two 2p AOs that overlap along the bond. It is high in energy at-0.58753 a.u., and wre valence AOs and so they are heavily involved in bonding.There is some sp mixing.
This shows the MO orbital formed from two 2p AOs that were perpendicular to the bond and so when they have overlapped this shape is produced. It is occupied and has an energy of -0.52332 a.u. They were valence orbitals and so are involved in chemical bonding. There is some sp mixing.