Rep:Mod:Everyone's At It
Module 2: Inorganic Chemistry
Ammonia Borane
Before my own mini project I was interested to run some brief calculations on ammonia borane as this molecule is potentially a very important compound for the future. In the modern world, where we find ourselves more consious of the potential consequences of our actions it has become increasingly apparent that we face an impending fuel crisis. As such the world of science is scrambling to find alternative ways of generating energy. One of the most promising ideas is the use of hydrogen as a fuel, this is a fantastic idea with a vast array of benefits, though this requires the discovery of effective and efficient methods by which to store hydrogen.
One such idea is the use of Ammonia Borane, which has 19.6% Hydrogen by weight as well as being both solid, and stable at ambient temperature. This makes it a highly plausible solution. The aim of this brief study is to investigate Ammonia Borane.
Firstly the actual structure can be investigated. This could of course be staggered or eclipsed and by computational studies one can predict what the ground state structure would be. We would expect from convention that the molecule would be staggered due to the high favourability of anti-periplanar bonds.
Peforming initial calculations allowed us to quickly elucidate that the computationally favourable configuration was the staggered structure. This was proven by taking both structures and optimising them to find that the lower energy was the staggered structure, as given belowDOI:10042/to-1845 :
| ||||||
This staggered conformation is observed when optimising from a number of structures including the structure optimising to the staggered conformation from the eclipsed conformation. This can be explained when we look at the energy difference of the two conformers. In doing so we see that the energy of the eclipsed structure is -82.75499074 Hartree, compared with -82.76661791 Hartree, which is an energy difference of approximately 31kJ mol-1. This explains why the optimisation to the staggered conformation is so favourable.
We can compare this with ethane which has a similar structure and so an identical possibility of being either staggered or eclipsed. In ethane this energy difference between the staggered and the less favourable eclipsed form is around 13kJ mol-1, less than half of the value for Ammonia Borane.
One possible explanation for this is the type of bonding present in Ammonia Borane. As nitrogen is a very electronegative species and boron an electro positive species we can see that this may have a direct effect on the electron distribution in the molecule, seeing the electron density on boron being pulled towards nitrogen and resulting in what are effectively ionic species. This is in stark contrast to ethane, in which electronegativity is almost entirely negligible due to the almost zero difference in the polarities of the constituent atoms, resulting in wholly covalent species.
This is an effect which GaussView has displayed, if we look at the 3D representation of the molecule we see no bond between boron and nitrogen which can be shown to reflect the highly ionic nature of the bonds. This is in fact a very useful property of the system as this high dipolar moment is in fact what allows AB to be found as a solid at room temperature which is clearly vital to its use as a hydrogen store.
We can take this difference in energy from structures one step further and move to an even more extreme case. This is a related molecule in Methyl Ammonia Borane. This has received less attention due to it's reduced hydrogen content by weight, more than 10% less! One would expect the energy difference here to be larger due to the increased steric bulk from the methyl groups.
The two summary files are shown below:
| MeAB Conformational Study | |||
| Staggered MeAB | Eclipsed MeAB | ||

|
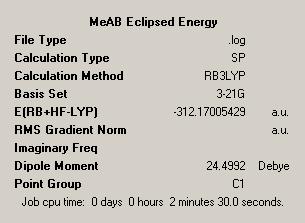
| ||
The energy difference can be seen to be 0.02039484 Hartree and so approximately 54kJ mol-1. The chosen explanation for this is the increased steric bulk of the methyl groups adding to the favourability of the staggered conformation with anti-periplanar bonds. We can from this be more confident in the assertion that the staggered conformation is chosen for Ammonia Borane and related compounds.
Trends Within A Group: Group 2
Trends within groups are a fascinating string on the bow of inorganic chemistry. Trends are useful as they explain numerous phenomena and give chemists an opportunity to develop rules and theories which can be applied to predict and rationalise the results of a particular study.
So valuable are such trends that in 1998 they were the subject of a inorganic text "Essential Trends in Inorganic Chemistry" by D.M.P Mingos, formally of Imperial College. This is a widely used text, underlining the many uses of such trends.
In this study the trends within group 2, specifically Magnesium and Calcium, will be investigated to see the similarities and differences between compounds. To begin with we should consider the trends which occur down the group so we can look out for observations around these.
Firstly as we go down the group the atomic radii increases. This is due to the increased size of the higher atomic orbitals resulting in more diffuse orbitals and so a larger atomic size. Additionally the elements become more and more electropositive as we go down the group. In essence the main difference between the two atoms will be the atomic size and so the difference in the size of the orbitals. The qualitative effect of this will be an interesting point.
The molecule which has been chosen for optimisation and study is MN(SiH3)2(μ-N(SiH3))2 where M in this case is either Ca or Mg. If we use the above trends as a guideline for our expected observations then we would expect to see a number of key facts in the optimised molecules. Firstly we would expect to see longer bond lengths for Ca than Mg due to its larger and more diffuse ionic radius and atomic size. We might also expect to see some evidence of the difference in electronegativity between the two atoms though this is unlikely to be huge as both are fairly electropositive.
The first report of this molecule was the report of the calcium species in 1991[1] and since then molecules and related species have been prevalent in the literature in a number of fields. These have included possible uses as heavy grignard reagents[2] as well as studies of 3-centre, 2-electron bonds[3]. The original paper included discussion of how this molecule was stabilised and this included agostic interactions. These could be the reason why a molecule such as this which does not immediately appear stable could be stabilised enough to be isolated.
The first method employed was a similar process to that which was used for previous optimisations was employed. The relevent structures were drawn and generated in GaussView. These structures were then optimised simply to move towards the fully optimised structure. As such the DFT method with a B3LYP hybrid functional and a 3-21G basis set was used. This however resulted in an error, this was because I had failed to consider the atoms involved. The models used work well for the first row elements but are less good for the lower elements. It was therefore a requirement that we use psuedopotentials to get a good approximation.
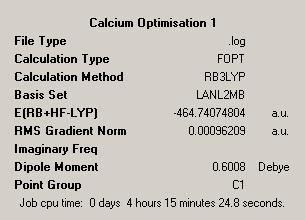
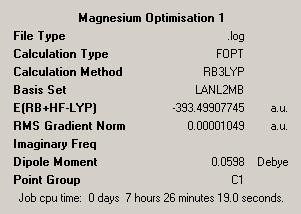
As such a similar process to that followed for the transition metals was used. This began with a low accuracy optimisation with a loose convergence and an LANL2MB psuedopotential, this would ensure that the calculation reached convergence. This gave the below structures for CalciumDOI:10042/to-1911 and MagnesiumDOI:10042/to-1912 :
| Initial Optimisations | |||
| Calcium | Magnesium | ||
 |
 | ||
 |
 | ||
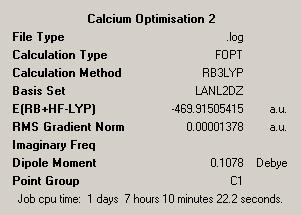
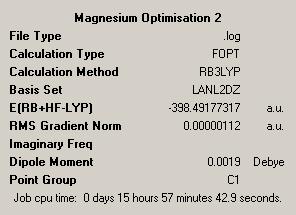
As had been done before these structures were then optimised further using a more accurate psuedopotential, that of LANL2DZ approximation. This is better than the previous approximation as it uses the more complex DZ basis set compared to the MB basis set from before, again the results are given below:
| Second Optimisations | |||
| CalciumDOI:10042/to-1914 | MagnesiumDOI:10042/to-1915 | ||
|
| ||
|
| ||
In order to ensure these structures were fully optimised an even more accurate technique was employed. This was MP2 modelling with an LANL2DZ basis set. These calculations were left to run for over two days but failed to converge. This might have been expected due to the large size of both the molecule and its constituent atoms. This would have potentially allowed us to generate a more accurate energy for the compound by giving us insight into the electronic distribution around the molecule however in view of the short amount of time available the computational cost of such a calculation was deemed too high.
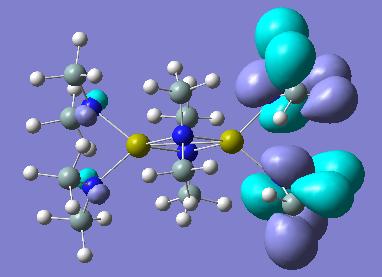
We could however gain a more qualitative view of the electron distribution by looking at the key molecular orbitals in these two molecule. Pictorial representations are given in the below table:
| Molecular Orbitals | |||
| Calcium HOMO | Magnesium HOMO | ||
|
| ||
| Calcium LUMO + 1 | Magnesium LUMO + 1 | ||

|
| ||
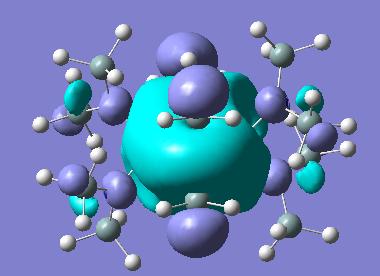
In looking at the HOMO orbitals of both molecules we see that the majority of the HOMO orbital density is based on the terminal N(SiH3)2 groups. This suggests that this is where we find any electron density that could be donated nucleophilically. This makes sense as the N atoms do have lone pair which they could donate.
The LUMO orbital in both cases is the inverse of the HOMO orbital and is degenerate in energy with the HOMO, this is in line with the idea that this structure is somewhat electron deficient, consistent with the need to form 3-centre, 2-electron bridging bonds as seen in the molecule.
The LUMO + 1 orbital is in fact interesting and spans the entire of the bridging units. These are located on a part of the molecule which is very electron deficient and so the fact that these are spanned by a diffuse molecular orbital suggests the fact that these orbitals, and so the declocalised electrons which make up the bonds, are very susceptible to nucleophilic attack. This is not unexpected as the bridging units are very strained as they require sp3 hybridised nitrogen atoms which are unfavourable as well as sp3 hybrid metal centres. The attack of a nucleophile to break the bridging bonds is as such a process which could be investigated.
This is something that has been researched in the literature to good effect and has also helped to highlight these molecules as similar to Grignard reagents forming an equilibrium with solvation with different solvents in solution, examples of which have been solvation with donating solvents such as ethers or THF.
Moving forward the results of the optimisations give us structures which we can now begin to compare to literature in order to look at our results. Literature around this issue is rife, with a key author in this field being Matthias Westerhausen who reported the original structure given earlier. He authored a review[4] on this general subject and this provides a huge amount of information with which we can compare our results. This comparison is given in the below table.
| Bond Lengths Comparison | |||||
| Parameter | LANL2MB | LANL2DZ | Literature | ||
| Ca-N (terminal) | 2.38 | 2.32 | 2.27 | ||
| Ca-N (bridging) | 2.52 | 2.5 | 2.47 | ||
| N-Si (terminal) | 1.84 | 1.77 | 1.7 | ||
| N-Si (bridging) | 1.89 | 1.78 | 1.7 | ||
| Shortest H-Ca | 3.36 | 2.51 | N/A | ||
The same can be done for the magnesium bond lengths:
| Bond Lengths Comparison | |||||
| Parameter | LANL2MB | LANL2DZ | Literature | ||
| Mg-N (terminal) | 2.06 | 1.98 | 1.98 | ||
| Mg-N (bridging) | 2.09 | 2.13 | 2.15 | ||
| N-Si (terminal) | 1.84 | 1.77 | 1.7 | ||
| N-Si (bridging) | 1.88 | 1.80 | 1.7 | ||
| Shortest H-Mg | 3.19 | 2.41 | N/A | ||
The first comparison we can make is a direct one between the two compounds. It is clear we see longer bond lengths at all points, both computationally and in literature, for the Calcium analogue compared to the Magnesium compound. This was as expected due to the larger atomic radius of calcium in comparison to magnesium, resulting in a more diffuse orbitals which give less good overlap and so longer bonds between the atoms.
We see two different bond lengths for the M-N terminal and M-N bridging bonds in all cases. This is a useful observation but explains a key phenomena in these molecules. The M-N terminal bonds are similar to M-N bonds which are given in general for other M-N systems, this suggests these are simple bonds, which is correct as they are terminal bonds.
In contrast the bridging M-N bonds are longer than these, longer bonds are indicative of less electron density between the two atoms. This is consistent with the fact that these are bridging bonds and so by definition have to donate electron density to two bonds. These are simply 3 centre 2 electron bonds and so the electron density is spread over more atoms than normal, so less electron density is found between the atoms and so we see longer bonds.
Two different bond lengths are also noticed for the N-Si bonds. The bridging bonds are longer than the terminal bonds. Again we can consider this from an electron density point of view. The bridging bonds are longer which again suggests a smaller amount of electron density in the bridging bonds. We can consider this by electron density from Nitrogen being used for the bridging bonds meaning a smaller amount of electron density is available for the N-Si bonds and so these are longer.
The final length we have denoted is the shortest M-H distance. These are deemed important as in literature reports of analogues with N(SiMe3)2 report an agostic interaction with the methyl groups. An agostic interaction is generally characterised by a short distance between the metal and a hydrogen atom, usually in the range of 1.8-2.3Å and is observed in the NMR Spectrum. In both of the systems we do see short M-H interactions and so we can see the agostic interaction. Such interactions are useful as they are observed in catalytic systems.
Agostic interactions are useful as they confer stability to the system with the stabilisation generally regarded as about 10-15kcal mol-1 and so these interactions are good as they reduce the total energy of the system. This may explain why we can stabilise the formation of bridging bonds. These agostic interactions are a very key factor in conferring stability to the molecule, as can be demonstrated later with the chlorinated analogues.
We can note the comparison between the literature and the computational values and so we see that the validity of the data is likely to be high due to the agreement of our data with that reported in the literature.
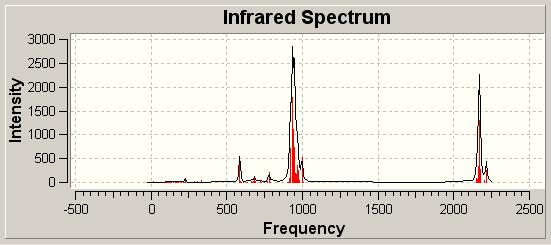
To study further the strength of the bonds it was possible to run a frequency analysis of the compounds generated to look at the vibrations of the molecule and the overall spectrum to allow comparisons between the two to be drawn. These spectra are given in the below table:
| Tabulated IR Spectra | |||||
| Ca IR Spectrum DOI:10042/to-1979 | |||||
| |||||
| Mg IR Spectrum DOI:10042/to-1978 | |||||

| |||||
We can tabulate this IR data and then compare this with the data from literature[5]
| Literature IR Comparison | |||||
| Ca | Mg | ||||
| Frequency | Literature | Stretch | Frequency | Literature | Stretch |
| 983 | 1010 | Si-N-Si Terminal | 943 | 970 | Si-N-Si Terminal |
| 899 | 920 | Si-N-Si Bridging | 922 | 955 | Si-N-Si Bridging |
| 348 | 410 | Ca-N Terminal | 438 | 465 | Mg-N Terminal |
| 330 | 410 | Ca-N Bridging | 408 | 465 | Mg-N Bridging |
The IR spectrum is very useful in order to further extend what we discussed earlier. We see obviously a number of stretches for the numerous Si-H bonds throughout the molecule. In addition to this there are 4 key stretches.
We see 2 different stretches in the spectrum for the N-Si bonds. This is because the two different types of bonds have different strengths and so are observed at different frequency. The terminal N atoms have more electron density with which to bond to the Si and so we see a stronger bond and hence a higher frequency. In contrast the bridging N atoms have less electron density with which to contribute to the N-Si bond and so we find a weaker bond and lower frequency. The situation can be considered from a different point of view in that the terminal nitrogen atoms have a p-lone pair which can donate into the d-orbitals on Silicon, leading to a strengthening of the N-Si bond. As the bridging nitrogen atoms have no such free lone pair they are unable to undergo this back bonding. This leads to no increase in strength of the bond and so a longer bond length, and weaker bond.
A similar pattern is seen for the M-N bonds which have two frequencies. A higher one for the terminal bond compared to the bridging bond.
We notice that the strength of the M-N bond decreases for Calcium compared to Magnesium. This can be explained as the heavy metal atom has larger orbitals and so forms a smaller, hence weaker, overlap with the nitrogen. This means that the bond is weaker, this is observed in the spectrum.
The fit of the computational data to the literature is good and so we are satisfied with our results.
Chlorine Analogues
After the SiH3 compounds had been looked into it was decided to investigate effect of swapping these for SiCl3 groups. It was expected that the effect of this would be a loss of the agostic interaction we saw in the previous compounds.
Again the initial optimisation was done with LANL2MB psuedopotential.
| Initial Optimisations | |||
| Calcium[3] | Magnesium[4] | ||
 |
 | ||
Again we follow this with a higher accuracy method of the LANL2DZ.
| Second Optimisations | |||
| CalciumDOI:10042/to-1985 | MagnesiumDOI:10042/to-1986 | ||
|
| ||
We can look at the bond lengths and compare these again to the literature values from before:
| Bond Lengths Comparison | |||||
| Parameter | LANL2MB | LANL2DZ | Literature | ||
| Ca-N (terminal) | 1.95 | 2.75 | 2.27 | ||
| Ca-N (bridging) | 2.00 | 2.62 | 2.47 | ||
| N-Si (terminal) | 1.85 | 1.73 | 1.7 | ||
| N-Si (bridging) | 1.94 | 1.78 | 1.7 | ||
| Shortest Cl-Ca | 6.03 | 5.12 | N/A | ||
| Bond Lengths Comparison | |||||
| Parameter | LANL2MB | LANL2DZ | Literature | ||
| Mg-N (terminal) | 1.85 | 1.95 | 1.98 | ||
| Mg-N (bridging) | 1.92 | 1.98 | 2.15 | ||
| N-Si (terminal) | 1.85 | 1.83 | 1.7 | ||
| N-Si (bridging) | 1.99 | 2.01 | 1.7 | ||
| Shortest Cl-Mg | 4.71 | 4.10 | N/A | ||
As before we see a fairly good correlation between the computational data and the literature reports.
We again see two different types of M-N bonds. These are terminal and bridging and these have a direct result on the N-Si bond lengths, which is again seen in the reports.
We have lost the previous agostic interaction as had been expected due to the replacement of H with Cl. This has a direct effect on the compound as the stability is now reduced due to the loss of this stabilising interaction. This has really shown that the agostic interactions in the original molecule are a key factor in conferring stability to a structure which did not immediately seem stable. The removal of these anomeric interactions seems to be enough to make bridging and formation of such dimeric molecules unfavourable.
The chlorinated analogues can be seen as much less stable due to the increased bond lengths in the compound and so these will be much more difficult to form, stabilise and react further suggesting this may not be a useful exercise in chemistry.
Conclusion
From this mini project we have been able to study the systems involved and noticed the trends down a group. We have seen by bond lengths and IR data the two different environments present in the molecule in terms of the amide groups.
Possible extentions of this work could include modelling the Lithium species to see if the diagonal trend between Li and Mg holds for these molecules. If more time was available this would be a simple process to investigate and could lead to definate results.
In the literature extentions have been much more speculative and quite ambitious with much chemistry of the Lanthanide group being done. The reason for this is clear, Ca is found in the 3+ state and the Ln3+ state is for most the sole stable state.
Much work has been done in this field with some very interesting results. These would be much more difficult to study computationally due to the difficulty in finding a psuedopotential which will take into account the f-orbitals which are buried close to the nucleus in the case of all of the lanthanides.
In summary this rewarding study has allowed us to see the different effects of the metal atom size as well as to study what would basically have been referred to as 3-centre, 2-electron bond which bridge the molecule we have studied. In this we have investigated the electronic and bonding effects of such bridging and have found very pleasing results giving us a better understanding of how the bridging occurs.
Finally in an extention of the study we have looked at the chlorinated analogues and found these to be much less stable. This is a key example showing the importance of the agostic interactions present in the original molecules.
Mja06 13:36, 18 February 2009 (UTC)
References
- ↑ Zeitschrift für anorganische und allgemeine Chemie, 604, 1, 127-140DOI:10.1002/zaac.19916040116
- ↑ Coordination Chemistry Reviews, Volume 252, Issues 15-17, August 2008, Pages 1516-1531 [[1]]
- ↑ Inorganic Chemistry Communications, 2003, 6, 1, 23-25DOI:10.1016/S1387-7003(02)00680-9
- ↑ M. Westerhausen, Coordination Chemistry Reviews, 176, 1998, 157-210 [[2]]
- ↑ Inorganic Chemistry, 1991, 30, 1, 96-101 DOI:10.1021/ic00001a018
