Rep:Mod:Eb1613 Ex3
Page List
The Calculations Log files can be found here
For main Introduction/conclusion page click here
For Excerise 1 page click here
For Excerise 2 page click here
For Excerise 3 page click here
Third Year Computational Lab Transition States - Emily Brown
Exercise 3
(This section was asked for in PM6. It's not a problem, but it might be why you ran out of time Tam10 (talk) 12:00, 6 December 2016 (UTC))
Both cheletropic and Diels-Alder reactions are possible for the above reactants.
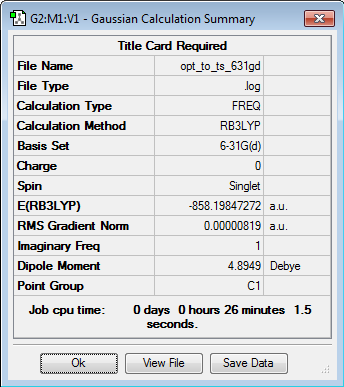
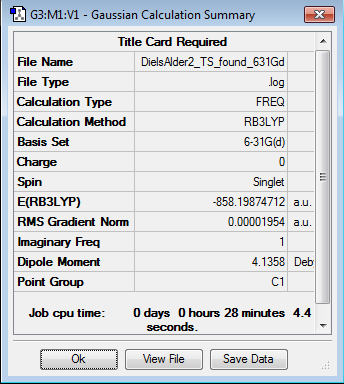
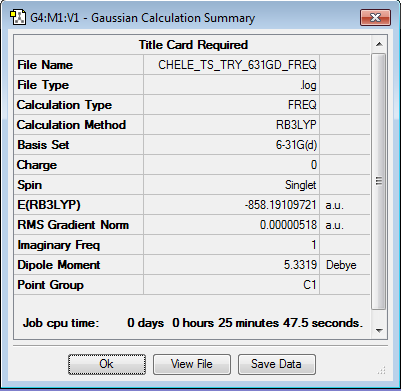
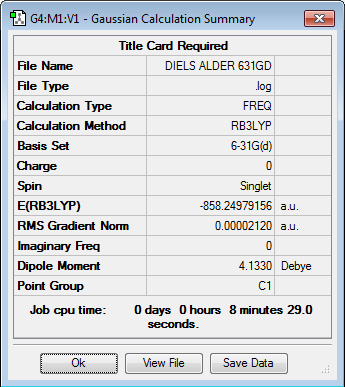
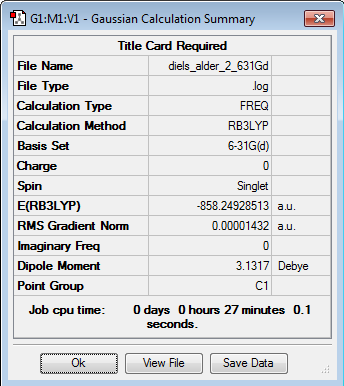
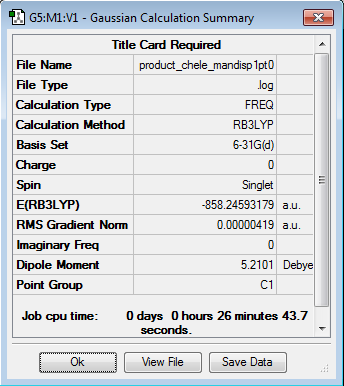
Initially the structures were optimized to the semi-empirical PM6 level and later the geometries were re-optimised to the DFT 6-31G(d)/b3LYP level of calculation. The reactants were aligned in different conformations and certain bonds were frozen to find the three transition states shown below. Every structure given has been optimized to the higher accuracy DFT 6-31G(d)/b3LYP level.
Unfortunately there was not enough time to investigate the other cis-butadiene fragment successfully.
Transition states
Below gif files are given to show the negative frequency vibration for the transition states which were located. The cheleotropic TS had the largest negative frequency values of the three.
| Exo Transition State. Negative Frequency = -198.65 cm-1 |
|---|
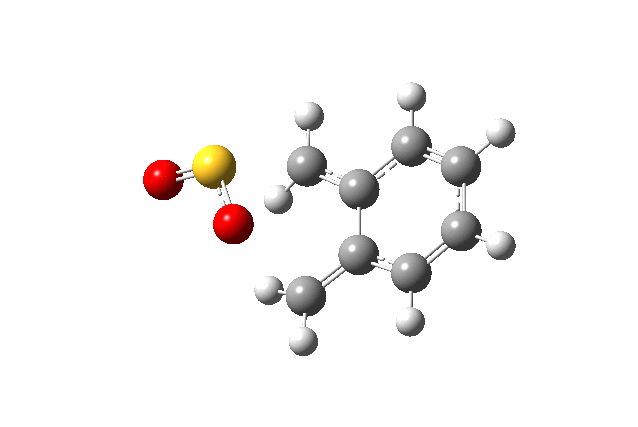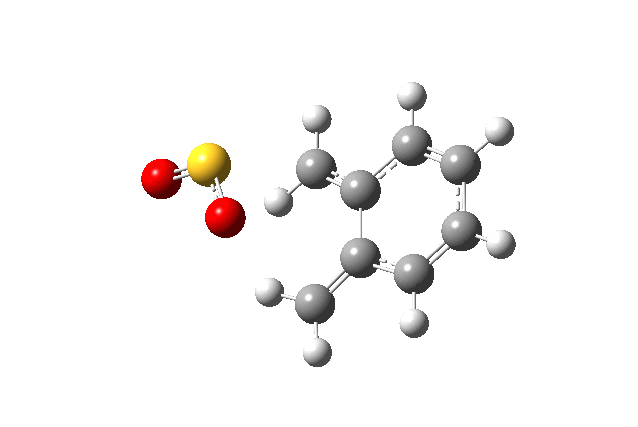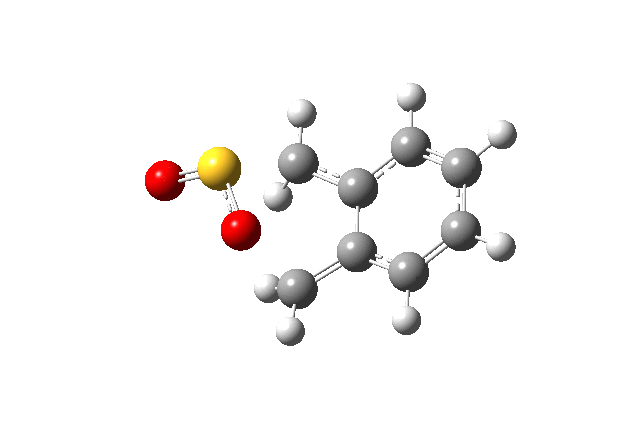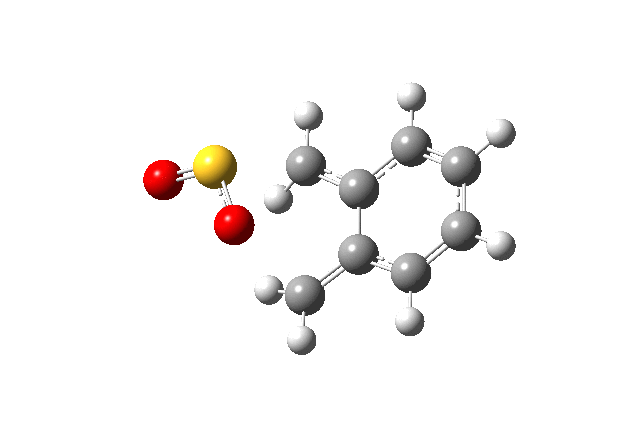
|
| Endo Transition State. Negative Frequency = -200.42 cm-1 |
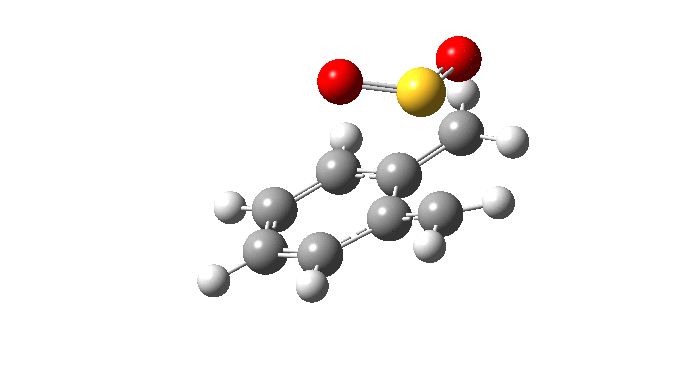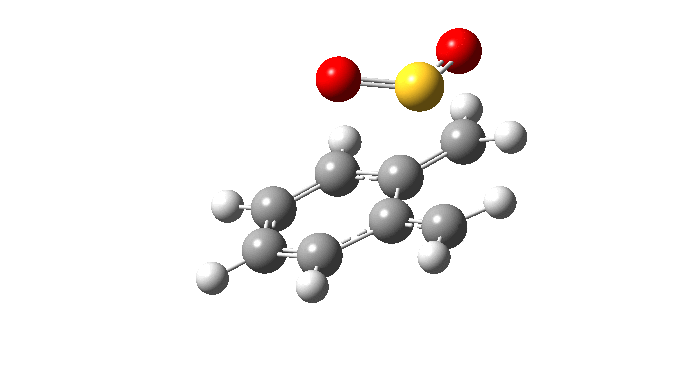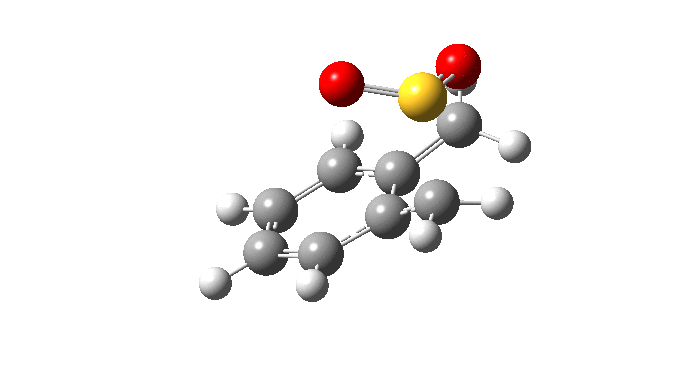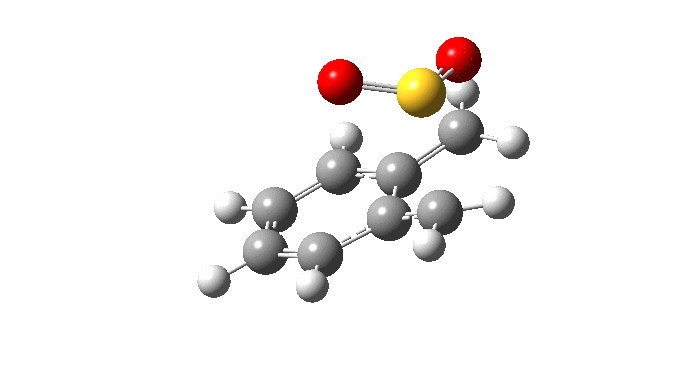
|
| Cheletropic Transition State. Negative Frequency = -221.18 cm-1 |

|
Thermochemistry anaylsis
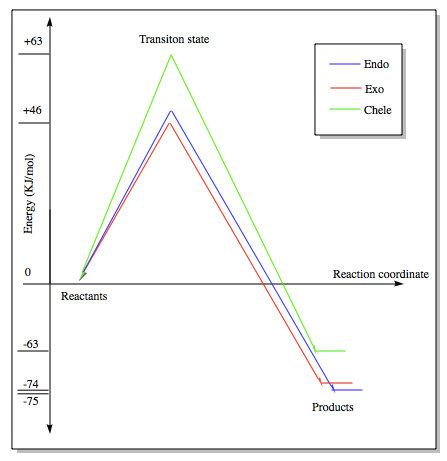
The energy profile was first plotted using excel and then using chemdraw to highlight the differences between the endo and exo paths. The endo-adduct has the lowest energy making it the thermodynamically preferred product. The cheleotropic transition state is of the highest energy and so is the least kinetically favored reaction path.
 |
|
(The diagram on the right shows the endo TS higher in energy than the exo. Are these energies the electronic energies or with thermal and entropic corrections? Tam10 (talk) 12:00, 6 December 2016 (UTC))
Energy values given relative to the reactants in KJ/mol, from the values the Endo route appears to be the preferred route.
| Reactants | Transition state | Products | |
|---|---|---|---|
| Endo | 0 | +46.17992 | -75.183818 |
| Exo | 0 | +46.23768 | -73.886821 |
| Cheletropic | 0 | +63.05663 | -63.9650565 |
Raw values with KJ/mol given to the nearest whole number
| Structure | E( Hartrees) | E(KJ/mol) |
|---|---|---|
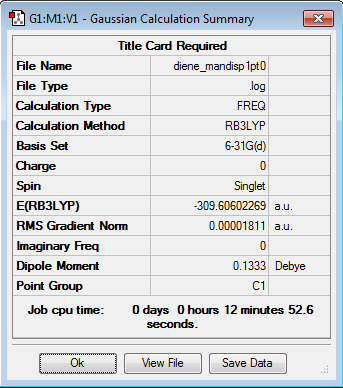
| Diene | -309.504492 | -812604 |
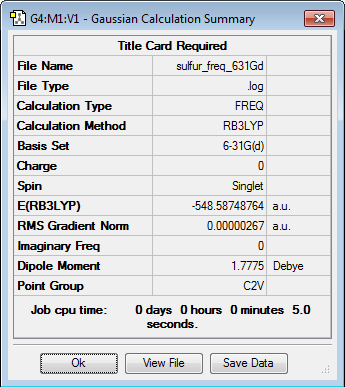
| Sulfur | -548.604917 | -1440362 |
| Sum of Reactants | -858.109409 | -2252966 |
| Endo TS | -858.09182 | -2252920 |
| Endo Product | -858.138045 | -2253041 |
| Exo TS | -858.091798 | -2252920 |
| Exo Product | -858.137551 | -2253040 |
| Chele TS | -858.085392 | -2252903 |
| Chele Product | -858.133772 | -2253030 |
(These energies look like the electronic energies with corrections. You should say where they come from Tam10 (talk) 12:00, 6 December 2016 (UTC))
Looking at the energy reaction barriers it can be seen that the Endo transition state is slightly below the cheletropic value making it the preferred reaction coordinate. however the value for the end transition state is only slightly below that for the cheletropic transition state and the calculation percentage errors/accuracies could effect this ordering.
IRC analysis
During the reaction the carbon-carbon bond lengths all become styled as 1.5 bond order suggesting an aromatic element to the ring. The IRC calculations proved very challenging throughout this lab and as such the ENDO TS IRC path is recorded in reverse and shows the detachment of the sulfur oxide molecule. Using the IRC plots it can be seen that the reaction proceed without any other intermediates or transition states as demonstrated by the smooth curve of the graph and lack of other turning points in the gradient.
(It's usual to end up with reversed IRCs. Gaussian doesn't know which way around 'reactants' and 'products' are Tam10 (talk) 12:00, 6 December 2016 (UTC))
| Cheletropic IRC path | |
|---|---|
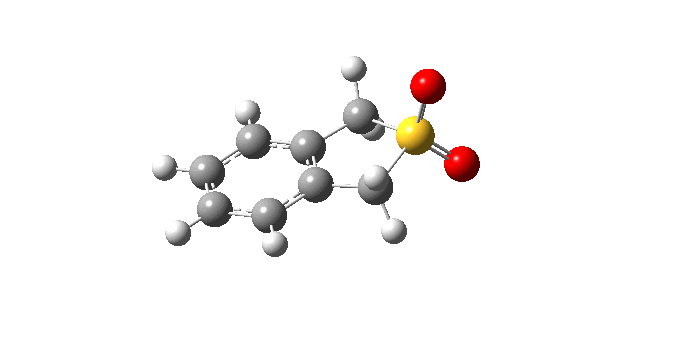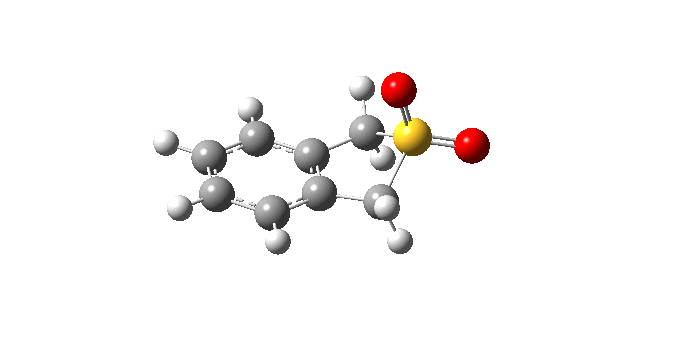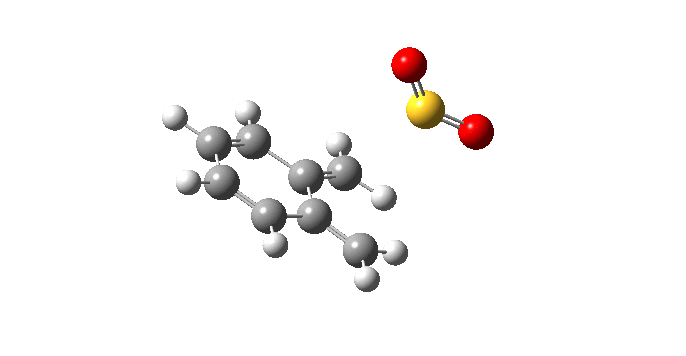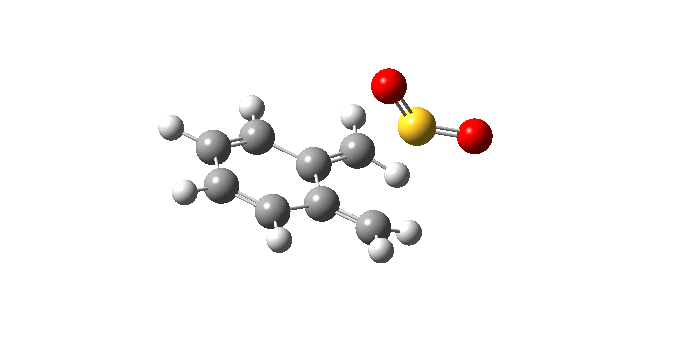 |
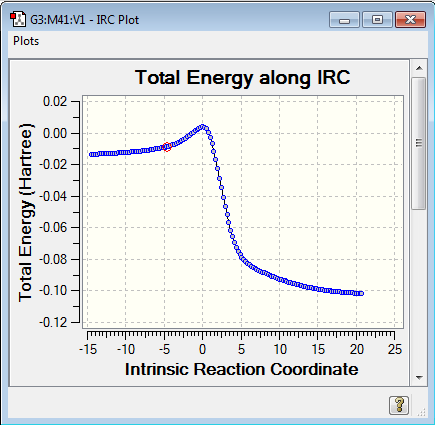
|
| Exo IRC path | |
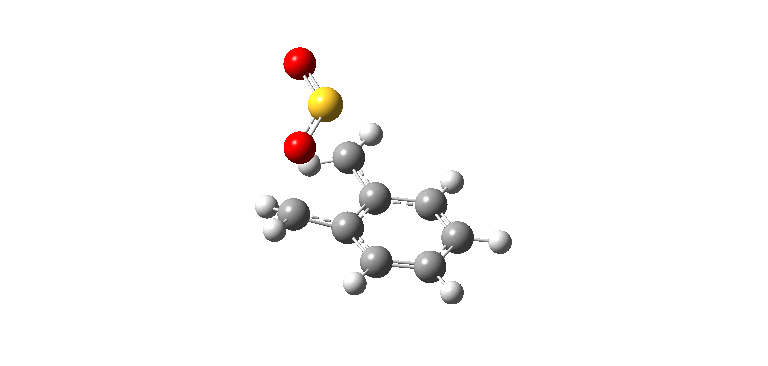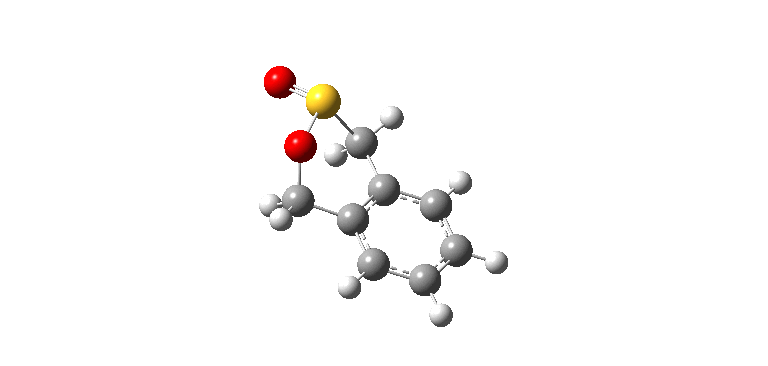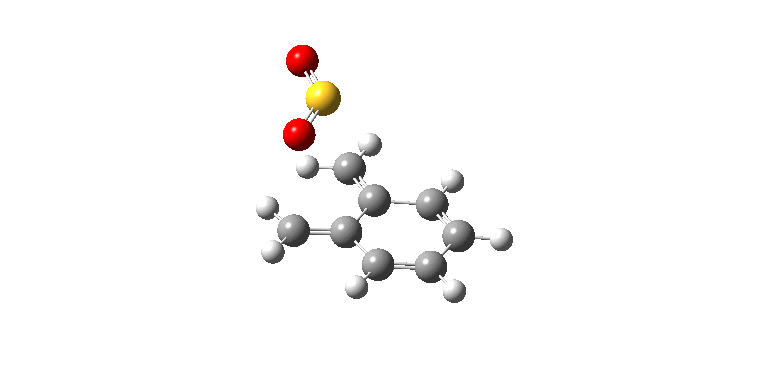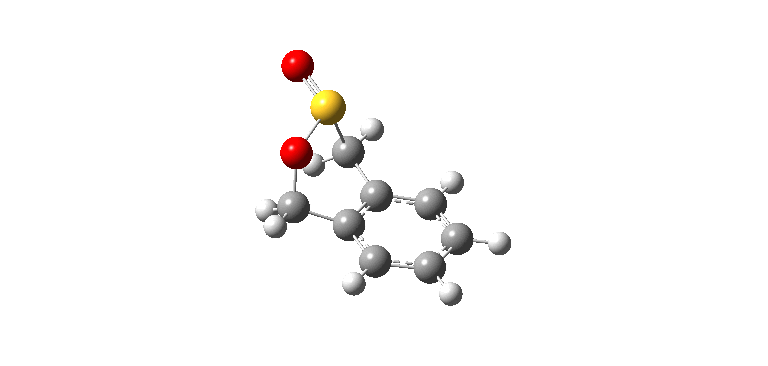 |
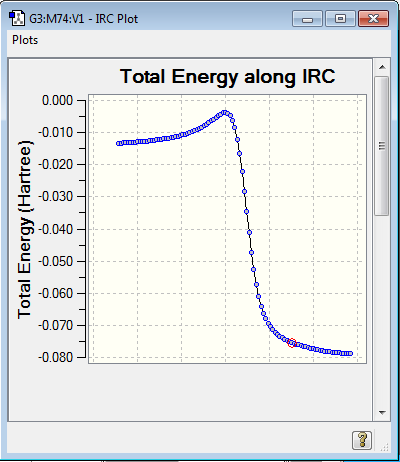
|
| Endo IRC path | |
 |

|