Rep:Mod:Eb1613 Ex2
Page List
The Calculations Log files can be found here
For main Introduction/conclusion page click here
For Excerise 1 page click here
For Excerise 2 page click here
For Excerise 3 page click here
Third Year Computational Lab Transition States - Emily Brown
Exercise 2
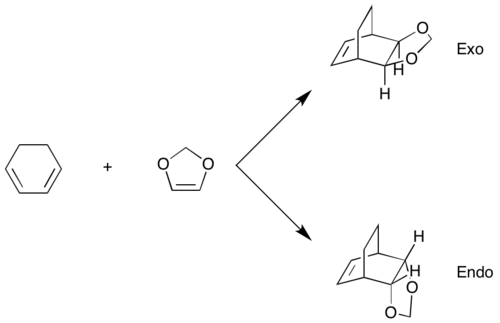
Two different products are known to form in this Diels-Alder reaction, they are known as the endo and exo adducts.
Transition state 2
These GIFs make it east to see where the Endo-TS has favorable secondary orbital interactions, towards the left hand side it can be seen that the oxygens p-oribtals could interact with those of the 6-carbon ring near it in a favorable manner.
(You have endo and exo mixed up. In the diagram above, it's shown correctly, but the figures and data below it's the wrong way around Tam10 (talk) 11:17, 6 December 2016 (UTC))
| Exo Transition State. Negative Frequency = -520.92 cm-1 |
|---|
|
| Endo Transition State. Negative Frequency = -528.82 cm-1 |

|
Thermochemistry anaylsis 2
Electronic and Thermal free energies relative to reactants(KJ/mol) (2dp)
| Reactants | Transition state | Products | |
|---|---|---|---|
| Exo | 0 | +159.81 | -67.41 |
| Endo | 0 | +167.65 | -63.77 |
From the thermochemistry relative energy values it can be seen that the Exo product has an activation energy which is 7.84 KJ/mol lower than that of the Exo product. The Exo-Adduct is 3.64 KJ/mol more stable than the Endo-adduct making it the thermodynamicly favoured product as well as the kinetically favoured product as it has a lower energy TS.
Nf710 (talk) 22:36, 14 December 2016 (UTC)Your energies are correct but you have got them the wrong way round.
MO analysis
Nf710 (talk) 22:45, 14 December 2016 (UTC) This setion was done fairly well however you got the orbital mixed up, howeve ryou missed a few things such as explicitly showing the secondary orbital overlap
This reaction and that the reaction in exercise 2 are both Diels-Alder reactions the MO diagram from exercise 1 is still valid to be used to understand/explain the MO interactions within this reaction.
| MO diagram for the Diels Alder Reaction in exercise 1 | |
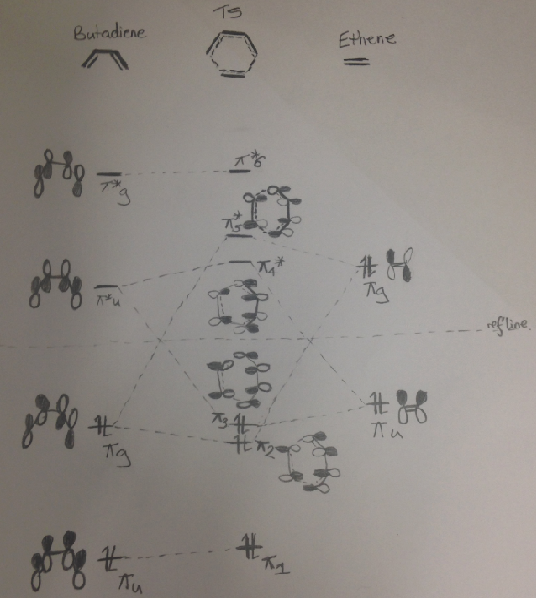
| |
(Butadiene has no centre of inversion and so cannot undergo the symmetry operation to determine gerade/ungerade. It's easier to look at the symmetry axis that bisects the TS Tam10 (talk) 11:35, 6 December 2016 (UTC))
From looking at the molcular orbitals below one can identify which orbitals are interacting in the Transition states. For example: it can be seen that the Endo TS LUMO is formed by the overlap of the Cyclohexadiene LUMO-1 and the 1,3-Dioxole LUMO.
Nf710 (talk) 22:46, 14 December 2016 (UTC) And this would suggest?
| MOs | |||
|---|---|---|---|
| Reactants | Transition States | ||
| 1,3-Dioxole | Cyclohexadiene | Endo-TS | Exo-TS |
| HOMO+1 | HOMO+1 | HOMO+1 | HOMO+1 |
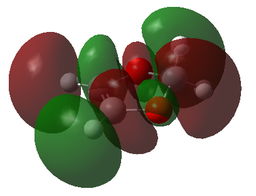 |
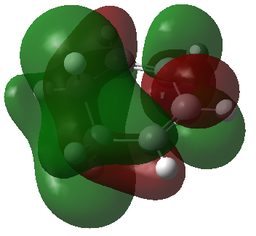 |
 |
|
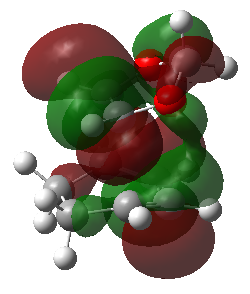
| HOMO | HOMO | HOMO | HOMO |
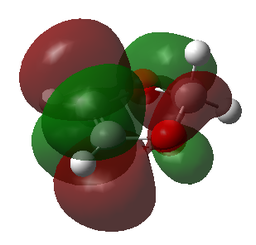 |
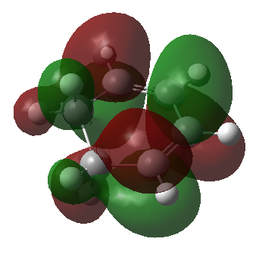 |
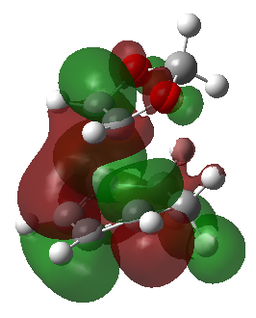 |
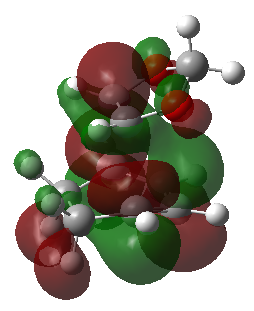
|
| LUMO | LUMO | LUMO | LUMO |
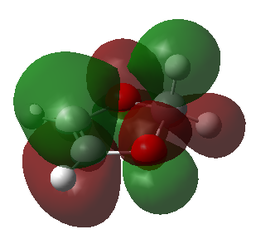 |
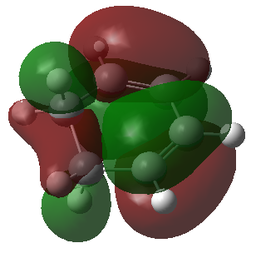 |
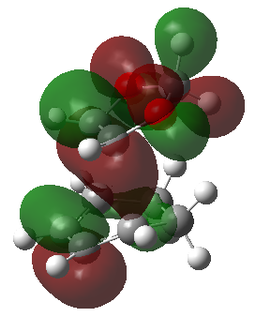 |
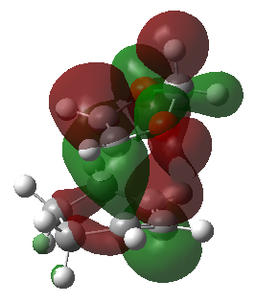
|
| LUMO-1 | LUMO-1 | LUMO-1 | LUMO-1 |
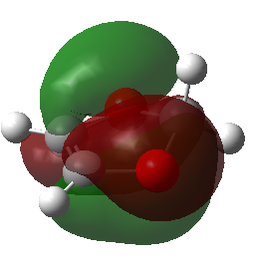 |
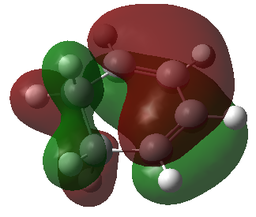 |
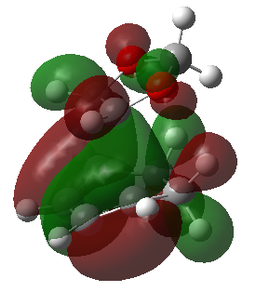 |
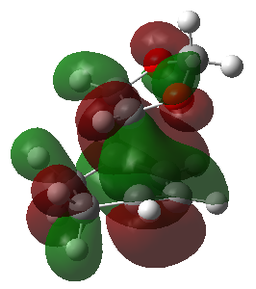
|
Structure Optimisation
All optimized to the B3LYP/6-31G(d) level but initially with the semi-empirical PM6 method. Everything but the transition states was confirmed to be a true minima by lack of negative frequencies in the frequency calculations as shown below. Finding a single negative frequency confirms the structures to in fact be the end and eco transition states desired.
| Cyclohexadiene | Optimization Summary | |||
|---|---|---|---|---|
|
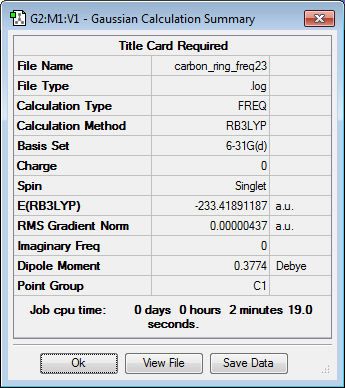
| |||
| 1,3-Dioxole | Optimization Summary | |||
|
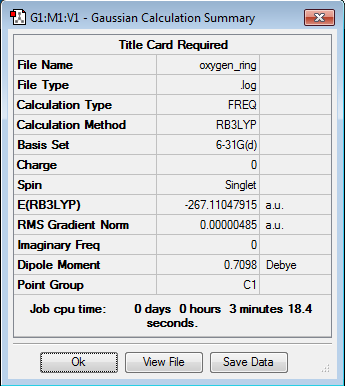
|
| Exo-TS | Optimization Summary | |||
|---|---|---|---|---|
|
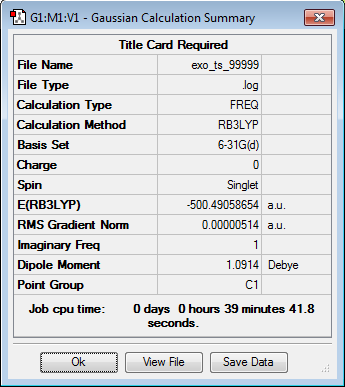
| |||
| Endo-Ts | Optimization Summary | |||
|
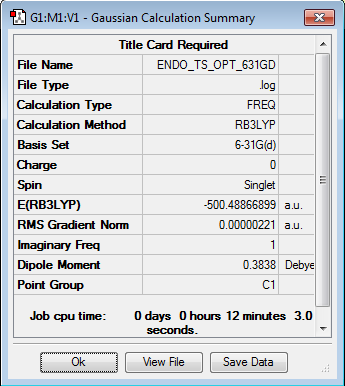
|
| Exo-Product | Optimization Summary | |||
|---|---|---|---|---|
|

| |||
| Endo-Product | Optimization Summary | |||
|
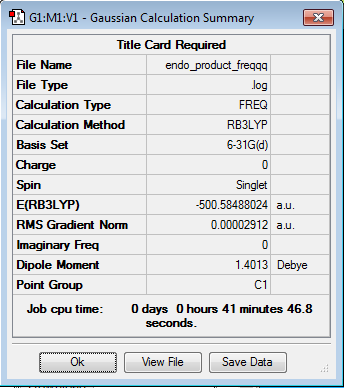
|