Rep:Mod:Eb1613 Ex1
Page List
The Calculations Log files can be found here
For main Introduction/conclusion page click here
For Excerise 1 page click here
For Excerise 2 page click here
For Excerise 3 page click here
Third Year Computational Lab Transition States - Emily Brown
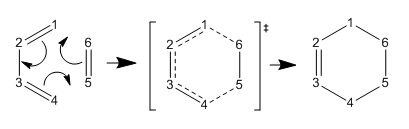
Exercise 1: Reaction of Butadiene with Ethene
Bond Distances
| C1-C2 | C2-C3 | C3-C4 | C4-C5 | C5-C6 | C6-C1 | |
|---|---|---|---|---|---|---|
| Butadiene | 1.33530 | 1.46838 | 1.33525 | |||
| TS | 1.37981 | 1.41112 | 1.37976 | 2.11496 | 1.38177 | 2.11452 |
| Product | 1.50086 | 1.33698 | 1.50086 | 1.53717 | 1.53459 | 1.53717 |
Carbon-Carbon Sigma bonds are longer than double bonds. the bond length between C1-C2 and C3-C4 increase as they transform from double to single bonds in the reaction. The C2-C3 bond length shortens due to the reaction as a double bond forms here. The C5-C6 bond lengthens as it transforms from a single to a double bond. Due to the system being within a ring the carbon carbon single bonds are slightly shorter than the literature values which is for alkane chains. The bond distances of the forming bonds C1-C6 and C4-C5 decrease to values corresponding to those of single bonds. The Van der Waals radi for carbon is 1.70 angstroms. [1] The length of bonds C1-C6 and C4-C5 at the transition state are less than twice the Van der Waals radius which indicates that a bond a forming.
Literature C-C bond distances [2]
| Carbon-Carbon Bond Distances (Angstroms) | |
|---|---|
| Sp3 C-C | Sp2 C-C |
| 1.57 | 1.33 |
MO analysis
It can be seen from the MOs that there is a high symmetry requirement for the reaction the HOMO-1 (v2) orbital interaction lowers the energy of the electrons from the diene; the HOMO (π3) interactions stabilizes the electron pair coming from the dieneophile.
The overlap integral is zero for the interactions labelled π3 and π*5 and non-zero for interactions labelled π2 and π*4.
The MOs diagram highlights that the reaction is allowed as the orbitals of the same parity are interacting: u<->u g<->g.
| MO diagram for the Diels Alder Reaction, self drawn. | |
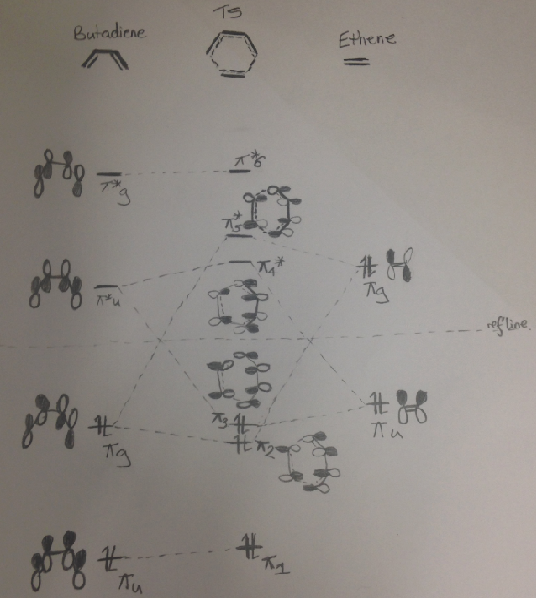
| |
Using a Frontier Orbital Approach to justify the diels alder reactivity in this reaction. This can be seen in the HOMO of the TS which shows the favorable overlap from combining the butadiene HOMO with the ethene LUMO.
| Butadiene | |
| HOMO | LUMO |
|---|---|
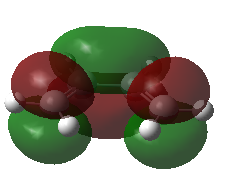 |
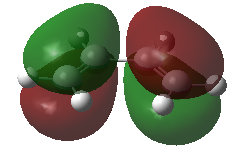
|
| Ethene | |
| HOMO | LUMO |
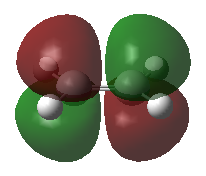 |
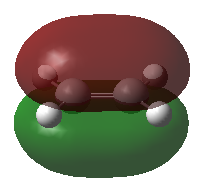
|
| Transition State | |
| LUMO + 1 = π*5 | |

| |
| LUMO = = π*4 | |
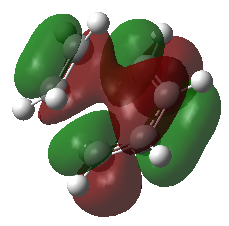
| |
| HOMO = = π3 | |
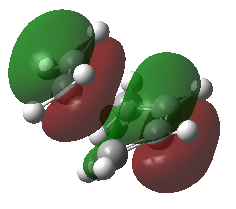
| |
| HOMO - 1 = = π2 | |
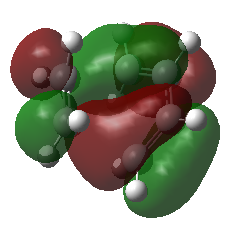
| |
The four MOs produced in the TS are π*5, π*4, π3 and π2. These are shown in the MO diagram above and were found were located using the guassian check point file.
Transition state analysis
The reactants were initially optimised to the PM6 semi-empirical level and then re-optimised to the DFT B3LYP/6-31G(d) level to save computational time. The reactants were aligned and then the atoms which form bonds were frozen using the opt=modredundant keywords in the calculation; this system was optimised to a minimum allowing parameters to reach a minimum whilst holding the reactants together at a distance of 2.2 angstroms. The resultant geometry from this calculation was used in a new calculation which optimised the structure to a TS (Berry) and allowed force constants to be calculated once. The transition state was located and confirmed by the presence of a single negative frequency which had an imaginary vibrational node which corresponded to the forming/breaking of bonds involved in the reactions transition state. This transition state geometry was then calculated using the higher DFT B3LYP/6-31G(d) calculation method. The transition state was further confirmed via the IRC calculated which allows force constants to be calculated always. The IRC requests that a reaction path be followed by integrating the intrinsic reaction coordinate. [3] It was found that using 10 steps in the IRC calculation was not nearly enough to look over the whole reaction coordinate and so 10000 steps was used for the repeated calculation. The visualisation of the transition state can be seen in the GIF files below along with the IRC calculation results. Furthermore the intrinsic reaction coordinate graph shows that there are no intermediates or other transition states involved in this reaction.
| IRC movie captured | IRC Graph |
|---|---|
 |
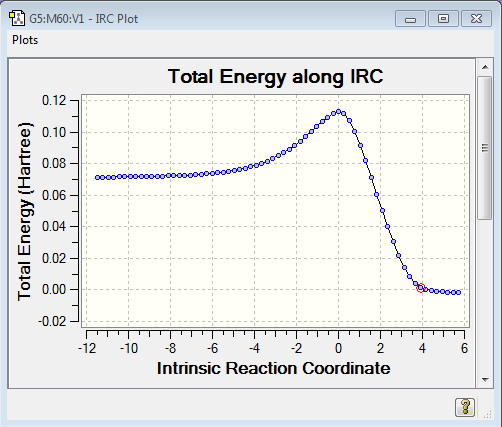
|
The Diels-Alder cyclic addition reaction occurs via a concerted transition state as shown below. The two new sigma bonds are formed at the same time therefore the reaction is synchronous. The animations below show how this compares to the first positive frequency, which is seen to be asynchronous rocking.
| Negative Frequency = -948.76 cm-1 |
|---|
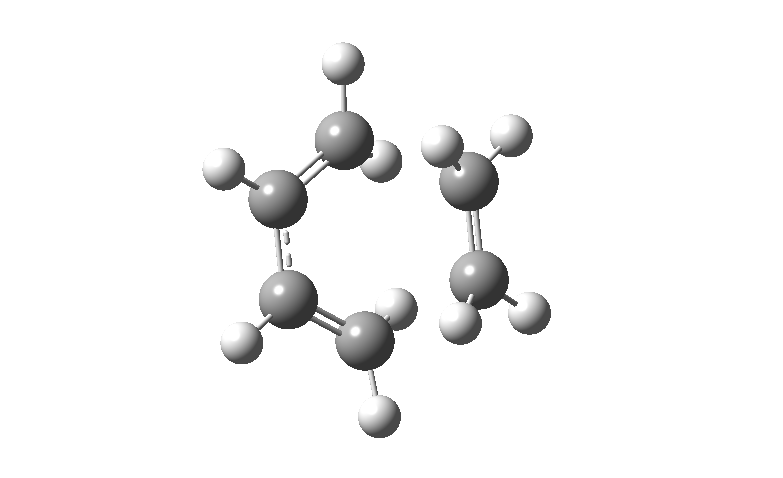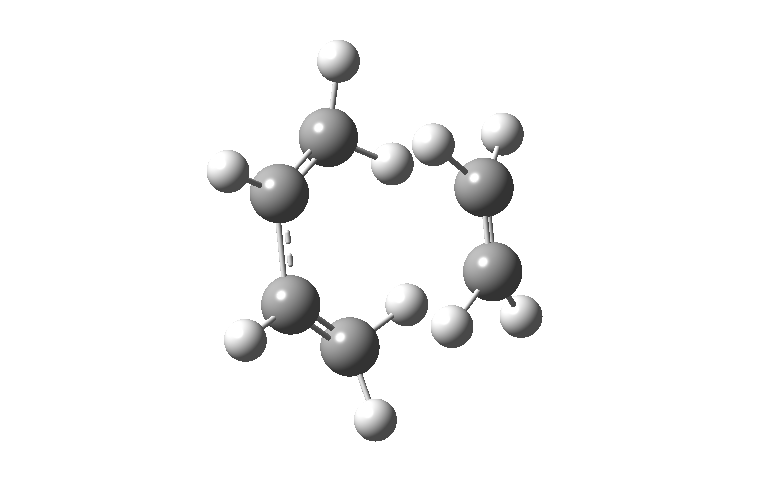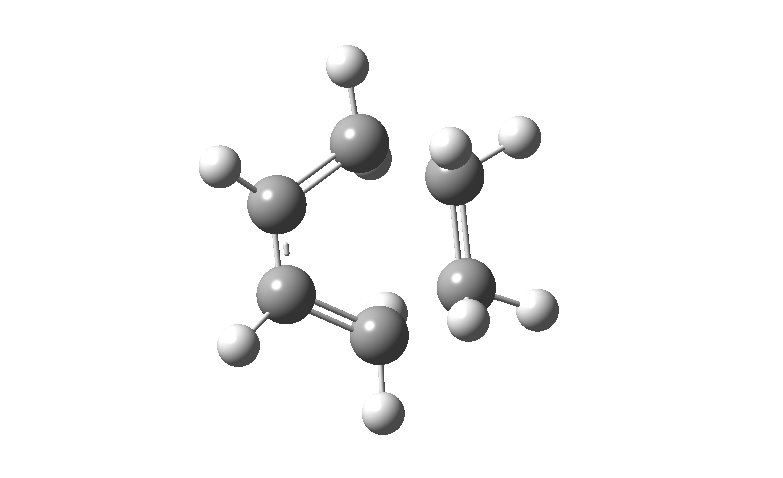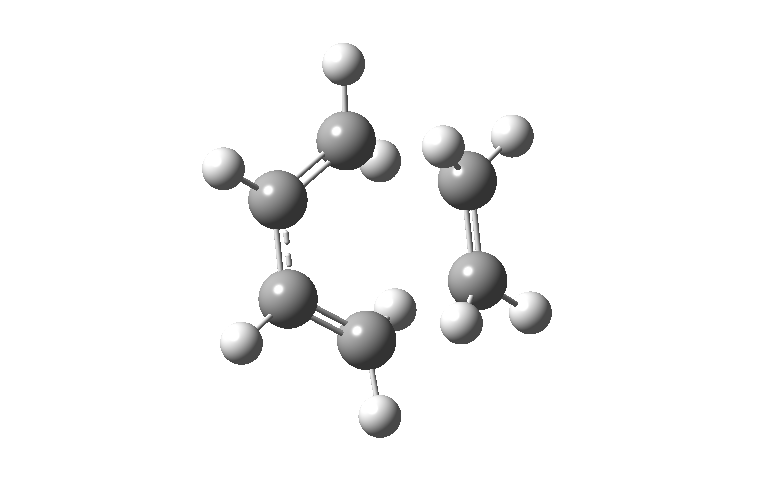
|
| First Positive Frequency = 145.08 cm-1 |
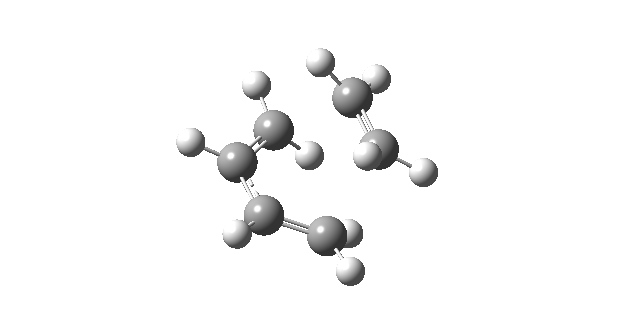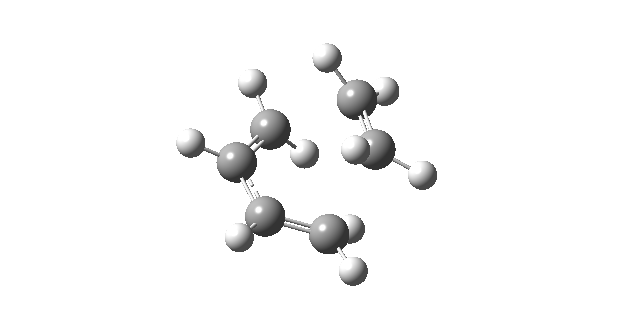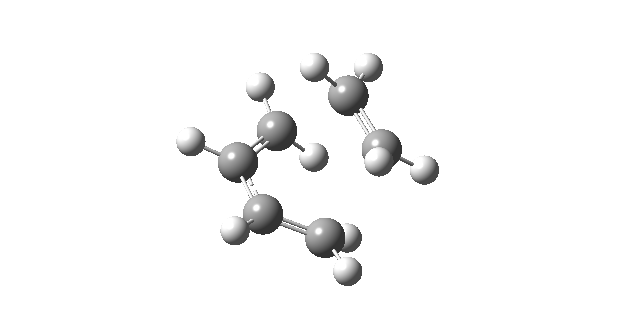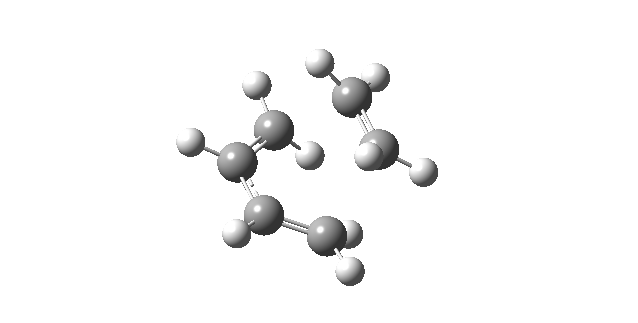
|
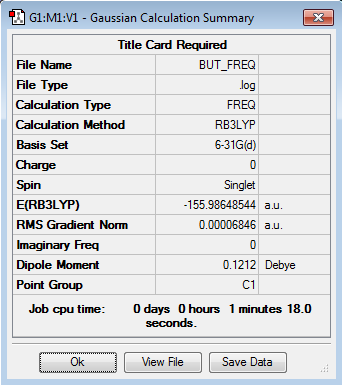
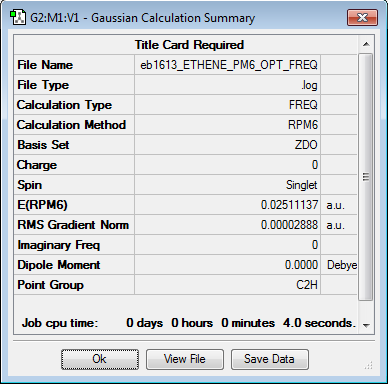
Optimization of Reactants, TS and Products
All structures have been optimised at the semi-empirical PM6 level and then further optimised to the DFT B3LYP/6-31G(d) level to save computational time. The lack of negative frequencies in the frequency calculation indicates that the structure is optimised to a true minimum, the presence of one negative frequency indicates that a transition state has been reached, more than one negative frequency means that a saddle point structure has been reached.
Nf710 (talk) 22:31, 14 December 2016 (UTC) Good section everything answered fairly well. nicely presented.
References
- ↑ A. Bondi, J. Phys. Chem., 1964, 68 (3), 441-451.
- ↑ J.M. Berg, J. L. Tymoczko, L. Stryer, in Biochemistry., W.H. Freeman, New York, 5th edn., 2002, Appendix C: Standard Bond Lengths, https://www.ncbi.nlm.nih.gov/books/NBK21220/, (accessed 23rd November, 2016).
- ↑ http://www.gaussian.com/g_tech/g_ur/k_irc.htm (accessed 23rd November, 2016)