Rep:Mod:ERMGERD
Module 1
Modelling Using Molecular Mechanics
The Hydrogenation of Cyclopentadiene Dimer
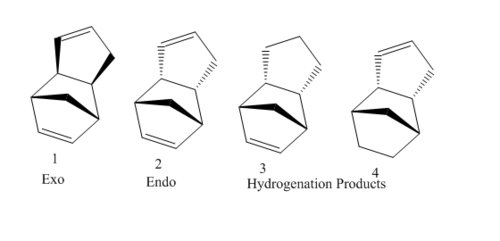
Cyclopentadiene dimerises into the two dimers, shown right, using the molecular mechanics technique the dimers are optimised to their minimum energy in ChemBio3D. It is known that the endo product is preferred and the major product from the dimerisation but is this controlled kinetically or thermodynamically?
Comparison of the MM2 optimised dimers are tabulated below. The endo dimer can be hydrogenated initially producing a dihydro derivative, the two possible derivatives shown right, MM2 calculations also allows inspection of these molecules and which one if the thermodynmaically stable derivative.
| Property | Exo | Endo | Dihydro 3 | Dihydro 4 |
|---|---|---|---|---|
| Stretch | 1.2850 | 1.2507 | 1.2352 | 1.0972 |
| Bend | 20.5783 | 20.8476 | 18.9388 | 14.5237 |
| Stretch-bend | -0.8382 | -0.8358 | -0.7609 | -0.5497 |
| Torsion | 7.6559 | 9.5109 | 12.1235 | 12.4968 |
| 1,4 VDW | 4.2346 | 4.3195 | 5.7288 | 4.5132 |
| Dipole/Dipole | 0.3775 | 0.4476 | 0.1631 | 0.1406 |
| Total energy | 31.8765 kcal/mol | 33.9975 kcal/mol | 35.9266 kcal/mol | 31.1520 kcal/mol |
The above table shows that the Exo dimer is actually the thermodynamic product meaning that the dimerisation is under kinetic control if endo is the major product, comparing the two dimers it is clear that the endo is favored because of its increased dipole/dipole interactions as well as a lesser bend. Dihydro 4 is the most thermodynamically stabled and if reaction is not kinetically controlled will be the major product, less stretch-bend and bend contribute to its stabilization however it does have more torsion strain than dihyrdo 3.
Atropisomerism in an Intermediate Related to the Synthesis of Taxol
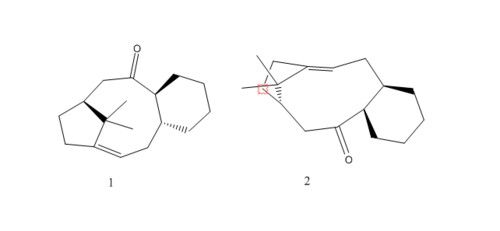
A key intermediate for the anti-cancer drug Taxol is shown on the right in its two atropisomers in the total synthesis of the drug the carbonyl is shown either up or down in which is apparently isomerises to the alternative carbonyl isomer, the most stable. Using ChemBio3D the most stable atropisomer can be found.
| Property | MM2(1) | MMFF94(1) | MM2(2) | MMFF94(2) |
|---|---|---|---|---|
| Stretch | 2.4652 | 2.6381 | ||
| Bend | 12.3805 | 12.5604 | ||
| Stretch-bend | 0.2131 | 0.4253 | ||
| Torsion | 16.2292 | 19.1193 | ||
| 1,4 VDW | 12.4518 | 12.8595 | ||
| Dipole/Dipole | -1.5851 | -1.7018 | ||
| Total energy | 40.1481 kcal/mol | 60.0679 kcal/mol | 44.2887 kcal/mol | 63.2835 kcal/mol |
Atropisomer(1) is shown to be the most stable in both the MM2 and MMFF94 calculations, however MMFF94 shows them at a higher energy and closer in value than the MM2 method.
Modelling Using Semi-empirical Molecular Orbital Theory
Regioselective Addition of Dichlorocarbene to a Diene
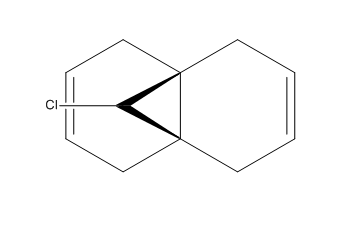
The above experiments have been of a classical, mechanical treatment of the molecule here a quantum mechanical method is used with the MOPAC calculation method. The diene molecule shown right is put under investigation probing its electrostatic potential, molecular orbitals and the vibrational frequency using Gaussian and also comparing the MOPAC method to the classical MM2.
A comparison of the MM2 and MOPAC calculation can be seen below in the .mol file, the structures look very similar but different straining in the center of the molecule and at the chloride atom, the results of the calculation are summarised below giving differing energies.
------------ Mopac Interface ------------ Model: georgedoucy_cl_diene.mol Mopac Job: AUX RM1 CHARGE=0 EF GNORM=0.100 SHIFT=80 Finished @ RMS Gradient = 0.08068 (< 0.10000) Heat of Formation = 22.82754 Kcal/Mol -----------------------------------------
------------MM2 Minimization------------ Warning: Some parameters are guessed (Quality = 1). Iteration 150: Minimization terminated normally because the gradient norm is less than the minimum gradient norm Stretch: 0.6185 Bend: 4.7328 Stretch-Bend: 0.0397 Torsion: 7.6627 Non-1,4 VDW: -1.0645 1,4 VDW: 5.7928 Dipole/Dipole: 0.1124 Total Energy: 17.8945 kcal/mol Calculation completed ------------------------------------
Georgedoucy cl diene comparision.mol
