Rep:Mod:EP01189257
Day 1ː Revision
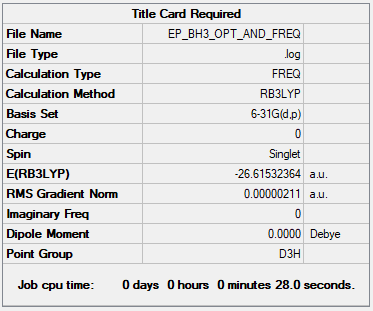
BH3
B3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.000017 0.001800 YES RMS Displacement 0.000011 0.001200 YES
Frequency file: EP BH3 OPT AND FREQ.LOG
Low frequencies --- -11.6892 -11.6814 -6.5475 -0.0014 0.0280 0.4290 Low frequencies --- 1162.9746 1213.1390 1213.1392
Optimised BH3 molecule |
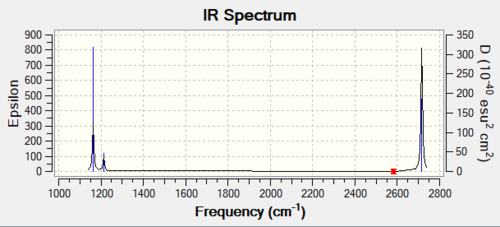
Vibrational spectrum for BH3
| Wavenumber (cm-1) | Intensity (arbitrary units) | Symmetry | IR active? | Type |
| 1163 | 93 | A2" | yes | out-of-plane bend |
| 1213 | 14 | E' | very slight | in-plane bend |
| 1213 | 14 | E' | very slight | in-plane bend |
| 2583 | 0 | A1' | no | symmetric stretch |
| 2716 | 126 | E' | yes | asymmetric stretch |
| 2716 | 126 | E' | yes | asymmetric stretch |
Explain why are there less than six peaks in the spectrum, when there are obviously six vibrationsː
Only 3 peaks are observed in the spectrum. Out of 6 vibrational modes, 5 are IR-active (1163, 1213, 1213, 2716, 2716 cm-1) because they induce a change in dipole moment. These 5 vibrational modes consists of two pairs of doubly degenerate modes (2716 & 1213 cm-1) and one non-degenerate mode (1163 cm-1), hence resulting in 3 peaks in spectrum.
The last one (2583 cm-1) is a symmetric stretch hence does not induce a change in dipole moment (vectors cancel one another) hence IR-inactive.
However, it is noteworthy that the peak at 1213 cm-1 has a low intensity, hence may not be experimentally observed.
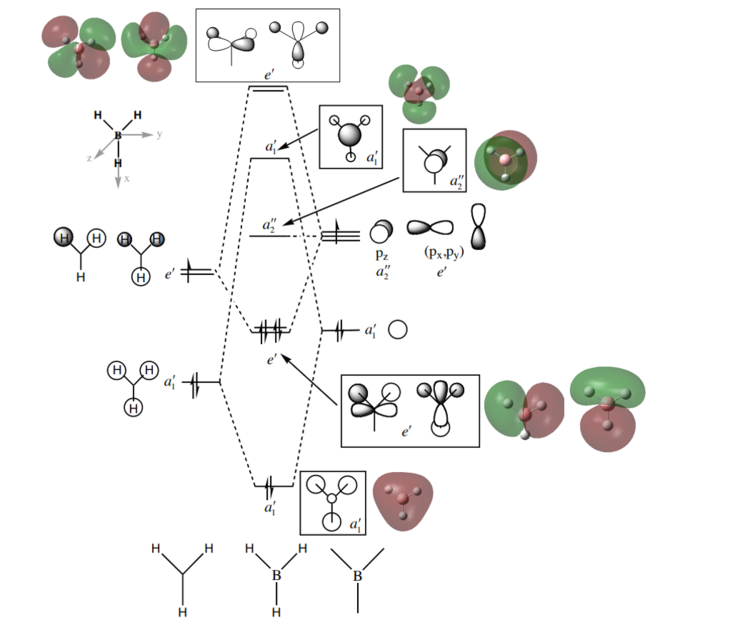
MO diagram of BH3
Diagram adapted from Hunt Research Group BH3 MO diagram1
Are there any significant differences between the real and LCAO MOs?ː
There are no significant differences between the real and LCAO MOs, except that the energy ordering for a1' and e' MOs are reversed. Also, unoccupied orbitals are more diffuse than occupied ones.
Ng611 (talk) 18:56, 8 May 2019 (BST) Is this something that qualitative MO theory does not predict?
What does this say about the accuracy and usefulness of qualitative MO theory?ː
This means that qualitative MO theory offers an accurate description of the MOs (in terms of MO shapes and relative energy ordering). However, qualitative diagrams are at best a rough approximation and should be cross-referenced with a more quantitative approach (running a calculation on Gaussian).
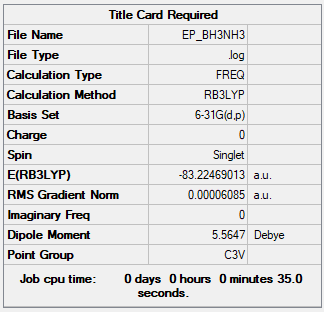
Association Energy (between NH3 and BH3)
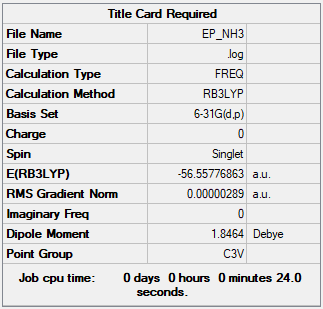
E(NH3) = -56.55777 a.u.
E(BH3) = -26.61532 a.u.
E(NH3BH3) = -83.22469 a.u.
ΔE = E(NH3BH3)-[E(NH3)+E(BH3)] = -0.05160 a.u. = -135 kJ/mol
Based on your energy calculation is the B-N dative bond weak, medium or strong? What comparison have you made to come to this conclusion?ː
A normal B-N covalent bond (as in borazine) is 389 kJ/mol.2 In comparison, a dative bond energy of 135 kJ/mol is weak.
However, from the perspective of a hydrogen bond (10 - 40 kJ/mol), this B-N dative bond (135 kJ/mol) is considered strong.
Ng611 (talk) 18:58, 8 May 2019 (BST) Correct calculation and correct comparison to the literature. Well done.

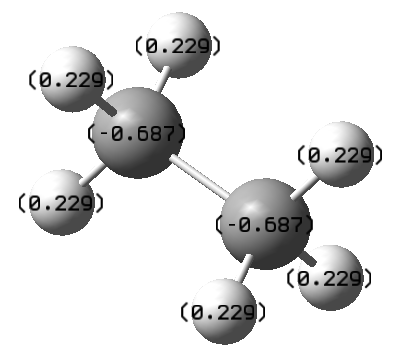
Optimise ethane and compute the NBO charges on the Carbon atoms, compute the NBO charges on the Nitrogen and Boron of aminoborane. Are these different? How does this relate to the character of the bond?ː
The NBO charges on C in ethane is -0.687 while B and N have NBO charges of -0.170 and -0.962, respectively. From this, we can tell that boron is more electron deficient than carbon while nitrogen is more electron rich than carbon. This results in a polar B-N bond in aminoborane as opposed to a non-polar C-C bond in ethane.
Charge on hydrogen atoms in both compoundsː
H atoms are positive (0.229) in ethane. On the other hand, H atoms on boron of ammonia-borane are negative (-0.059), making them hydridic in nature.
Dipole in both compoundsː
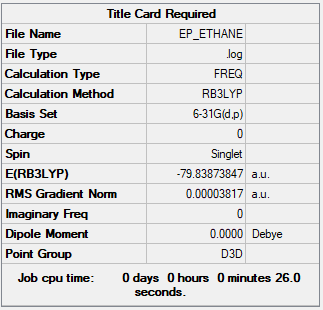
Dipole moment of ethane is zero, making it non-polar. On the other hand, ammonia-borane has dipole moment of 5.56 Debye, hence making it polar. This polarization allows for H-H interaction between hydridic H on one molecule and positively charged H on another molecule, resulting in formation of dihydrogen bonds (BHδ−···Hδ+N).3 An important consequence of this that ammonia-borane is a stable solid at room temperature with a high hydrogen content, making it a potential fuel.
How could you chemically manipulate the charges on the Carbon or Nitrogen or Boron atoms?ː
To manipulate the charge on C, N or B, one can install electron donating or withdrawing groups on them. For instance, the -NH3 can be replaced with -NF3 group, resulting in less electron rich nitrogen due to high electronegativity of fluorine. This has been reported to lower bond strength of B-N bond.4
NH3
B3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000005 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.000012 0.001800 YES RMS Displacement 0.000008 0.001200 YES
Frequency file: EP_NH3.LOG
Low frequencies --- -11.5223 -11.4866 -0.0032 0.0245 0.1415 25.6160 Low frequencies --- 1089.6618 1694.1735 1694.1738
Optimised NH3 molecule |
NH3BH3
B3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000124 0.000450 YES RMS Force 0.000057 0.000300 YES Maximum Displacement 0.000525 0.001800 YES RMS Displacement 0.000286 0.001200 YES
Frequency file: EP_BH3NH3.LOG
Low frequencies --- -0.0614 -0.0464 -0.0066 21.3718 21.3778 40.8284 Low frequencies --- 266.0565 632.3623 640.1180
Optimised NH3BH3 molecule |
Ethane
B3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000070 0.000450 YES RMS Force 0.000038 0.000300 YES Maximum Displacement 0.000214 0.001800 YES RMS Displacement 0.000149 0.001200 YES
Frequency file: EP_ETHANE.LOG
Low frequencies --- -19.6066 -19.5354 -0.0073 -0.0068 -0.0043 26.3240 Low frequencies --- 313.5371 827.7636 827.7638
Optimised ethane molecule |
Day 1ː New
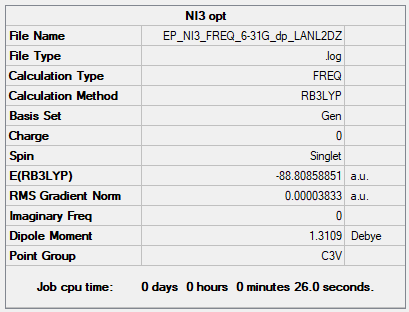
NI3
B3LYP/6-31G(d,p)LANL2DZ
Item Value Threshold Converged? Maximum Force 0.000064 0.000450 YES RMS Force 0.000038 0.000300 YES Maximum Displacement 0.000486 0.001800 YES RMS Displacement 0.000277 0.001200 YES
Frequency file: EP_NI3_FREQ_6-31G_dp_LANL2DZ.LOG
Low frequencies --- -12.7375 -12.7314 -6.2899 -0.0040 0.0188 0.0633 Low frequencies --- 101.0325 101.0332 147.4122
Optimised NI3 molecule |
Optimised N-I distanceː 2.184 Å (lit. valueː 2.18 Å)5
Noteː As this Gaussian calculation was performed locally in a computer lab instead of HPC, the file was not published on DSpace (authorized by Prof. Hunt).
Day 2/3ː Project
Ionic Liquids
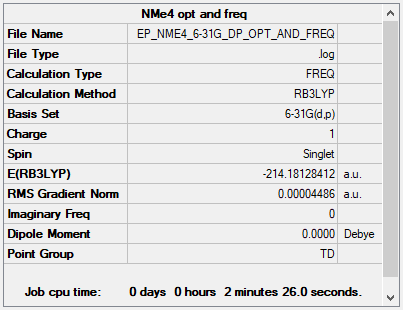
NMe4+
B3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000068 0.000450 YES RMS Force 0.000027 0.000300 YES Maximum Displacement 0.000155 0.001800 YES RMS Displacement 0.000067 0.001200 YES
Frequency file: EP_NME4_6-31G_DP_OPT_AND_FREQ.LOG
Low frequencies --- -0.0014 -0.0007 0.0002 22.7173 22.7173 22.7174 Low frequencies --- 190.7549 294.0686 294.0686
Optimised NMe4+ molecule |
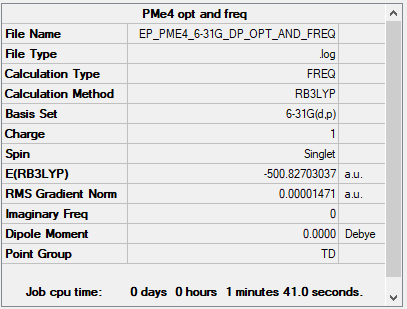
PMe4+
B3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000030 0.000450 YES RMS Force 0.000012 0.000300 YES Maximum Displacement 0.000110 0.001800 YES RMS Displacement 0.000046 0.001200 YES
Frequency file: EP_PME4_6-31G_DP_OPT_AND_FREQ.LOG
Low frequencies --- -0.0016 -0.0015 -0.0013 25.3085 25.3085 25.3086 Low frequencies --- 161.2545 195.7493 195.7493
Optimised PMe4+ molecule |
Comparison of NBO charges


| NMe4+ | PMe4+ |
| Nː -0.30 | P: 1.67 |
| C: -0.48 | C: -1.06 |
| H: 0.27 | Hː 0.30 |
Compute the MOs and carry out an NBO charge analysis (be sure you have NBO charges, not Mulliken!) for both structuresː
The charge of central atom is negative for NMe4+ (N: -0.30) and positive for PMe4+ (P: 1.67). This can be explained by the electronegativities of N (3.0) and P (2.1) relative to C (2.5). The high electronegativity of N results from the inductive electron withdrawal from the methyl groups in NMe4+, hence resulting in more positive C atom compared to PMe4+. Conversely, the lower electronegativity of P compared to C results in P being more positive than C.
Overall, the net positive charge on NMe4+ mainly lies on the peripheral H while the positive charge mainly sits on the central atom (P) for PMe4+.
Ng611 (talk) 19:25, 8 May 2019 (BST) Remember to discuss the effect of symmetry as well.
What does the "formal" positive charge on the N represent in the traditional picture? On what atoms is the positive charge actually located for this cation?ː
A formal charge is defined as the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of relative electronegativity.
The formal charge of any atom in a molecule can be calculated by the following equation:
where V is the number of valence electron of the neutral atom in isolation (in its ground state); N is the number of non-bonding valence electrons on this atom in the molecule; and B is the total number of electrons shared in bonds with other atoms in the molecule.
Traditionally, it means that the positive charge lies on the central N since it has lost an electron. However, a calculation revealed that the net positive charge on NMe4+ mainly lies on the peripheral H. This is due to the fact that N has high electronegativity, resulting in electron density being pulled towards the central atom, leaving the peripheral protons positive.
Ng611 (talk) 19:25, 8 May 2019 (BST) Interesting explanation. Well done.
MOs of NMe4+
| Molecular Orbital | LCAO MO diagram |
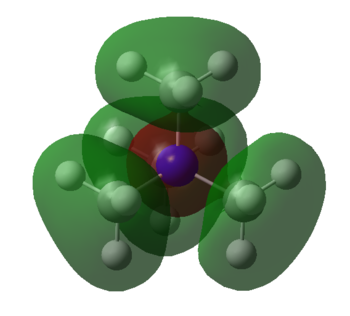
|

|

|
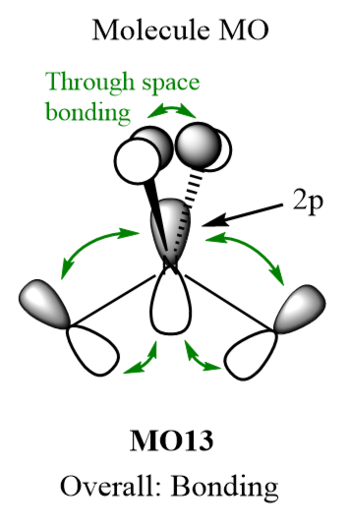
|
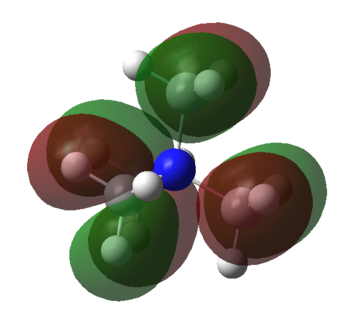
|
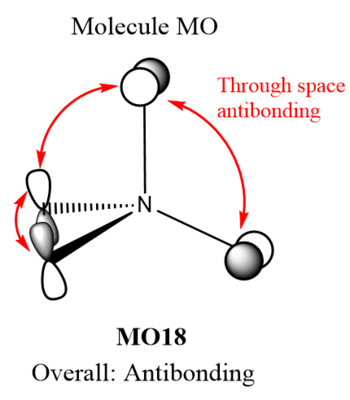
|
Smf115 Nice range of MOs selected in terms of complexity and your first one has been really well presented and annotated. However, the last two sadly don't contain the FOs which were needed.
Extension
Comment on or analyse or describe some aspect of this system which you found interestingː
The fact that N is more electronegative than P would lead one to expect that a proton on NMe4+ to be more positively charged than a proton on PMe4+.
However, NBO charges revealed that the opposite is true i.e. the H of PMe4+ is more positive compared to H of NMe4+.
Reasonː A possible explanation for this counter-intuitive result would beː A more electronegative N would lead to a more positive adjacent C in NMe4+. For charges to balance, the protons attached to this more positive charged C would have to be more negative, leading a less positive proton in NMe4+.
Smf115 Nice to see some optional analysis which supports the charge analysis section. Overall, very good wiki report!
References
1. Hunt, P. MO Diagram of BH3. Molecular Orbitals in Inorganic Chemistry Available at: http://www.huntresearchgroup.org.uk/teaching/teaching_comp_lab_year2a/Tut_MO_diagram_BH3.pdf (Accessed: 1st May 2019)
2. Cottrell, T. L. The strengths of chemical bonds. (Academic Press, 1961)
3. Aliev, A. E. & Harris, K. D. M. Probing Hydrogen Bonding in Solids Using Solid State NMR Spectroscopy. Supramolecular Assembly via Hydrogen Bonds I Structure and Bonding 1–53 (2003)
4. Fiorillo, A. A. & Galbraith, J. M. A Valence Bond Description of Coordinate Covalent Bonding. The Journal of Physical Chemistry A 108, 5126–5130 (2004)
5. Zhdankin, V. V. Iodine Heterocycles. Advances in Heterocyclic Chemistry 1–91 (2015)
Acknowledgements
I would like to extend my thanks to Prof. Hunt and Dr Doidge for their invaluable guidance and of course, the lab demonstrators who have offered constructive feedback on this report.