Rep:Mod:EFC18
efc18 wiki
NH3
Interactive 3D viewer
Summary table for optimised NH3 molecule
| Molecule | NH3 |
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Job type | Opt+Freq |
| E(RB3LYP) | -56.55776873 [a.u.] |
| RMS Gradient Norm | 0.00000323 [a.u.] |
| Point group | C3v |
| N-H bond length | 1.01798 [Å] (database[1] value 1.0124 [Å]) |
| H-N-H bond angle | 105.745 [°] (database[1] value 106.670 [°]) |
Item table
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000014 0.001800 YES
RMS Displacement 0.000009 0.001200 YES
Parameters being optimised
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.018 -DE/DX = 0.0 !
! R2 R(1,3) 1.018 -DE/DX = 0.0 !
! R3 R(1,4) 1.018 -DE/DX = 0.0 !
! A1 A(2,1,3) 105.7446 -DE/DX = 0.0 !
! A2 A(2,1,4) 105.7446 -DE/DX = 0.0 !
! A3 A(3,1,4) 105.7446 -DE/DX = 0.0 !
! D1 D(2,1,4,3) -111.8637 -DE/DX = 0.0 !
Display Vibrations window

NH3 vibrations data table
| Vibration | 1 | 2 | 3 | 4 | 5 | 6 |
| Wavenumber/cm-1 | 1090 | 1694 | 1694 | 3461 | 3590 | 3590 |
| Symmetry | A1 | E | E | A1 | E | E |
| Intensity/arbitrary units | 145 | 14 | 14 | 1 | 0 | 0 |
| Image | 
|

|

|

|

|

|
Questions about vibrations of NH3
- How many modes do you expect from the 3N-6 rule?
- I expect there to be 6 modes of vibration for this non-linear molecule using the 3N-6 rule and the fact that there are 4 atoms (N=4).
- Which modes are degenerate?
- Modes 2 and 3 have the same energy. Modes 5 and 6 also have the same energy, but this energy differs to that of modes 2 and 3
- Which modes are "bending" vibrations and which are "bond stretch" vibrations?
- Modes 1,2 and 3 are "bending" vibrations and modes 4,5 and 6 are "bond stretch" vibrations.
- Which mode is highly symmetric?
- Mode 4 is the most symmetric but mode 1 is also rather symmetric.
- One mode is known as the "umbrella" mode, which one is this?
- Mode 1.
- How many bands would you expect to see in an experimental spectrum of gaseous ammonia?
- 2 bands: one at frequency ~1700cm-1 due to H-N-H scissoring and another more intense band at frequency ~1100cm-1 due to N-H wagging.
Charge Analysis

The charge on the nitrogen atom is -1.125 and the charge on each hydrogen atom is +0.375. The fact that the sum of the charges equals zero is promising because ammonia is a neutral species overall. Another good sign is that the hydrogen atoms all have the same charge due to the C3 symmetry. However one might expect that the charge on nitrogen would be -3 and the charge on each hydrogen would be +1, but this basic model doesn't agree with the computed results.
N2
Interactive 3D viewer
Summary table for optimised N2 molecule
| Molecule | N2 |
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Job type | Opt+Freq |
| E(RB3LYP) | -109.52412868 [a.u.] |
| RMS Gradient Norm | 0.00000060 [a.u.] |
| Point group | D∞h |
| N-N bond length | 1.106 [Å] (database[2] value 1.098 [Å]) |
Item table
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Parameters being optimised
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1055 -DE/DX = 0.0 !
Display Vibrations window
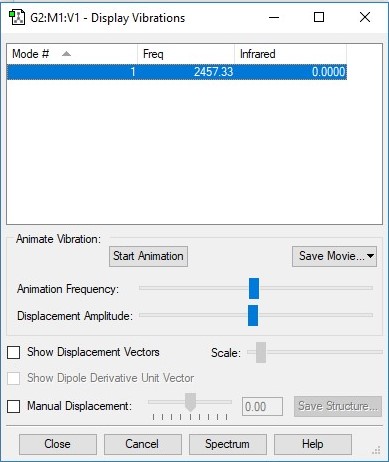
N2 vibrations data table
| Vibration | 1 |
| Wavenumber/cm-1 | 2457 |
| Symmetry | A1g |
| Intensity/arbitrary units | 0 |
| Image | 
|
N2 vibrations
I expect there to be only 1 vibrational mode for this linear molecule using the 3N-5 rule and the fact that there are 2 atoms (N=2). This mode is evidently a "bond stretch" vibration however we would not expect to observe this band in an experimental spectrum of gaseous N2 because this vibration doesn't induce a change in the molecule's dipole moment - something that is required of a vibration to make a molecule IR active (Δμ ≠ 0).
Charge Analysis

The charge on each nitrogen atom is 0 which is expected of this homonuclear diatomic molecule in its elemental form.
H2
Interactive 3D viewer
Summary table for optimised H2 molecule
| Molecule | H2 |
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Job type | Opt+Freq |
| E(RB3LYP) | -1.17853936 [a.u.] |
| RMS Gradient Norm | 0.00000017 [a.u.] |
| Point group | D∞h |
| H-H bond length | 0.743 [Å] (database[3] value 0.741 [Å]) |
Item table
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Parameters being optimised
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 0.7428 -DE/DX = 0.0 !
Display Vibrations window

H2 vibrations data table
| Vibration | 1 |
| Wavenumber/cm-1 | 4466 |
| Symmetry | A1g |
| Intensity/arbitrary units | 0 |
| Image | 
|
H2 vibrations
I expect there to be only 1 vibrational mode for this linear molecule using the 3N-5 rule and the fact that there are 2 atoms (N=2). This mode is evidently a "bond stretch" vibration however we would not expect to observe this band in an experimental spectrum of gaseous H2 because this vibration doesn't induce a change in the molecule's dipole moment - something that is required of a vibration to make a molecule IR active (Δμ ≠ 0).
Charge Analysis

The charge on each hydrogen atom is 0 which is expected of this homonuclear diatomic molecule in its elemental form.
Transition metal complex with N2
Unique REFCODE: BOWVUG
Transition metal complex with N2
Measured N-N bond length in complex: 1.125 [Å].
The two equivalent N-N bond lengths of 1.125 [Å] in this transition metal complex are both larger than that measured in elemental N2 (1.106 [Å]). This agrees with the fact that the N-N triple bond is partially weakened through coordination with the central metal ion and this results in a weaker, longer N-N triple bond in the complex.
Haber-Bosch calculations
| Energies | Value in [a.u] |
| E(NH3) | -56.55776873 |
| 2*E(NH3) | -113.11553746 |
| E(N2) | -109.52412868 |
| E(H2) | -1.17853936 |
| 3*E(H2) | -3.53561808 |
| ΔE | -0.0557907 |
Converting the energy difference from Hartrees [a.u] to kJmol-1 we obtain a ΔE value of -146.48 kJmol-1, which reveals that the product ammonia is more stable than the reactants hydrogen and nitrogen in the Haber-Bosch process.
NF3
Interactive 3D viewer
Summary table for optimised NF3 molecule
| Molecule | NF3 |
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Job type | Opt+Freq |
| E(RB3LYP) | -354.07131058 [a.u.] |
| RMS Gradient Norm | 0.00010257 [a.u.] |
| Point group | C3v |
| N-F bond length | 1.38404 [Å] (database[4] value 1.365 [Å]) |
| F-N-F bond angle | 101.830 [°] (database[4] value 102.37 [°]) |
Item table
Item Value Threshold Converged?
Maximum Force 0.000164 0.000450 YES
RMS Force 0.000108 0.000300 YES
Maximum Displacement 0.000613 0.001800 YES
RMS Displacement 0.000296 0.001200 YES
Parameters being optimised
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.384 -DE/DX = 0.0 !
! R2 R(1,3) 1.384 -DE/DX = 0.0 !
! R3 R(1,4) 1.384 -DE/DX = 0.0 !
! A1 A(2,1,3) 101.8302 -DE/DX = 0.0001 !
! A2 A(2,1,4) 101.8302 -DE/DX = 0.0002 !
! A3 A(3,1,4) 101.8302 -DE/DX = 0.0002 !
! D1 D(2,1,4,3) -104.9443 -DE/DX = -0.0001 !
Display Vibrations window
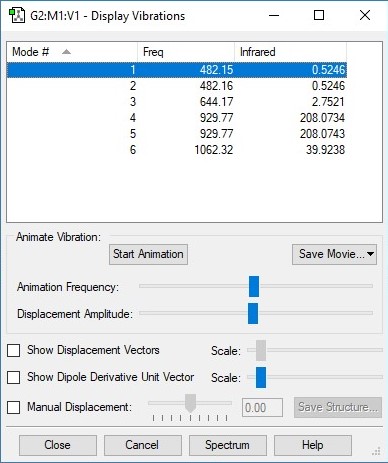
NF3 vibrations data table
| Vibration | 1 | 2 | 3 | 4 | 5 | 6 |
| Wavenumber/cm-1 | 644 | 482 | 482 | 1062 | 930 | 930 |
| Symmetry | A1 | E | E | A1 | E | E |
| Intensity/arbitrary units | 3 | 1 | 1 | 40 | 208 | 208 |
Questions about vibrations of NF3
- How many modes do you expect from the 3N-6 rule?
- I expect there to be 6 modes of vibration for this non-linear molecule using the 3N-6 rule and the fact that there are 4 atoms (N=4).
- Which modes are degenerate?
- Modes 2 and 3 have the same energy. Modes 5 and 6 also have the same energy, but this energy differs to that of modes 2 and 3
- Which modes are "bending" vibrations and which are "bond stretch" vibrations?
- Modes 2, 3, 5 and 6 are "bending" vibrations and modes 1 and 4 are "bond stretch" vibrations.
- Which mode is highly symmetric?
- Mode 4 is the most symmetric.
Charge Analysis

The charge on the nitrogen atom is +0.660 and the charge on each fluorine atom is -0.220. The fact that the sum of the charges equals zero is promising because NF3 is a neutral species overall. Another good sign is that the fluorine atoms all have the same charge due to the C3 symmetry. However one might expect that the charge on nitrogen would be +3 and the charge on each fluorine would be -1, but this basic model doesn't agree with the computed results.
Molecular orbitals of NF3

This MO has energy -1.35870 [a.u.]. This bonding MO is the result of a complete in-phase combination of the atomic 2s orbitals of nitrogen and fluorine.

This MO has energy -1.23341 [a.u.]. This anti-bonding MO is the result of an out-of-phase combination of the atomic 2s orbitals of two of the fluorine atoms.

This MO is the HOMO and has energy -0.35162 [a.u.]. This essentially non bonding MO is the result of 2p orbital from all the nitrogen and fluorine atoms, resulting in an orbital resembling a lone pair of electrons on nitrogen, which is in agreement with this molecule reacting as a lewis base and nucleophile through nitrogen.

This MO has energy -0.57102 [a.u.]. This anti-bonding MO is the result of an out-of-phase combination of the atomic 2p orbitals of nitrogen and fluorine.

This MO has energy -0.42224 [a.u.]. This anti-bonding MO is the result of a complete out-of phase combination of the atomic 2p orbitals of the 3 fluorines.
References
- ↑ 1.0 1.1 Computational Chemistry Comparison and Benchmark DataBase, http://cccbdb.nist.gov/exp2x.asp?casno=7664417, (accessed March 2019).
- ↑ Computational Chemistry Comparison and Benchmark DataBase, http://cccbdb.nist.gov/exp2x.asp?casno=7727379, (accessed March 2019).
- ↑ http://www.millsian.com/summarytables/SummaryTables022709S.pdf, (accessed March 2019).
- ↑ 4.0 4.1 Computational Chemistry Comparison and Benchmark DataBase, https://cccbdb.nist.gov/expgeom2.asp?casno=7783542&charge=0, (accessed March 2019).
Marking
Note: All grades and comments are provisional and subject to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have received your grade from blackboard.
Wiki structure and presentation 1/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES - very good structure with all necessary information easy to locate, well done!
Do you effectively use tables, figures and subheadings to communicate your work?
YES
NH3 1/1
Have you completed the calculation and given a link to the file?
YES
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES
N2 and H2 0.5/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
YES
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES
Have you compared your optimised bond distance to the crystal structure bond distance?
YES
Haber-Bosch reaction energy calculation 1/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
YES
Have you reported your answers to the correct number of decimal places?
YES
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES
Your choice of small molecule 4/5
Have you completed the calculation and included all relevant information?
YES
Have you added information about MOs and charges on atoms?
YES - you have laid out the information well however some of your explanations could be improved. You could have explained the charges with reference to the relative electronegativities of the elements. MO 17 is 2p from the F atoms, but 2s from the N atom. The MO is antibonding between N and F as there are nodal planes through each N-F bond. MO 11 is of primarily bonding character - it has a single nodal plane which runs through the atomic centres, not between them.
Independence 1/1
If you have finished everything else and have spare time in the lab you could: Check one of your results against the literature, or
Yes you have compared some of your results against literature values.
Do an extra calculation on another small molecule, or Do some deeper analysis on your results so far
