Rep:Mod:Dorene
Appearance
NH3
General information
| Molecule name | Calculation method | Basic set | Final energy E(RB3LYP) | RMS gradient | Point group | N-H bond distance | H-N-H bond angle |
|---|---|---|---|---|---|---|---|
| NH3 | RB3LYP | 6-31G(d,p) | -56,5577687299 a.u. | 0.05399560 a.u. | C3V | 1.01798Å | 105.741 |
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
J mol of NH3
test molecule |
Media:YONGYINCHEN_PHUNT_NH3_OPTF_POP.LOG
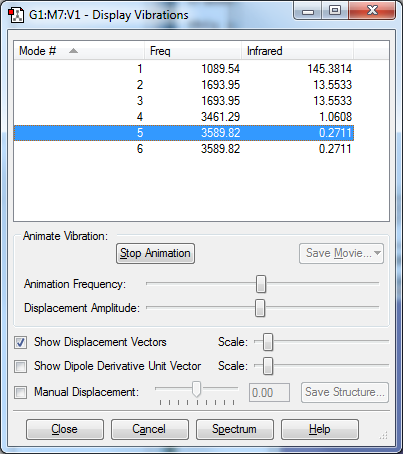
Vibration modes
• 6 modes are expected from the 3N-6 rule. • Modes 2&3 are degenerate to each other, so are modes 5&6. • Modes 1,2,3 are bending vibrations and modes 4,5,6 are bond stretch vibrations. • Mode 4 is highly symmetric, • Mode 1 is known as "umbrella" mode. • As vibrations of modes 4,5,6 do not have a change in dipole moment, they are not IR active. Therefore 2 bands would be seen in experimental spectrum
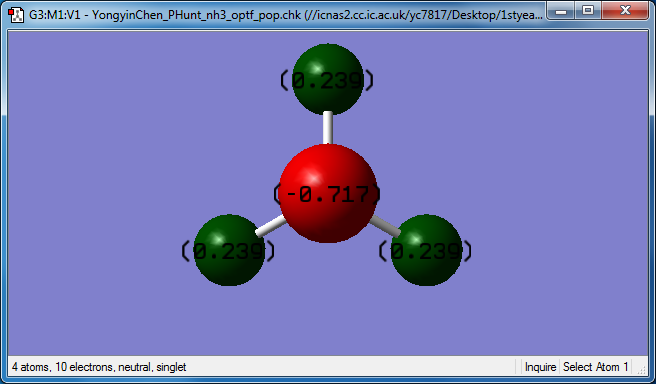
Charge of atoms
• N is more electronegative than H. Therefore, • N is expected to have a negative charge of -0.717 • H is expected to have a positive charge of +0.239
N2
General information
| Molecule name | Calculation method | Basic set | Final energy E(RB3LYP) | RMS gradient | Point group | N-N bond distance | N-N structure |
|---|---|---|---|---|---|---|---|
| N2 | RB3LYP | 6-31G(d,p) | -109.52412868 a.u. | 0.00000365 a.u. | D*H | 1.10550Å | Linear |
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000002 0.001800 YES RMS Displacement 0.000003 0.001200 YES
J mol of N2
test molecule |
Media:YONGYINCHEN_PHUNT_N2_OPTF_POP.LOG
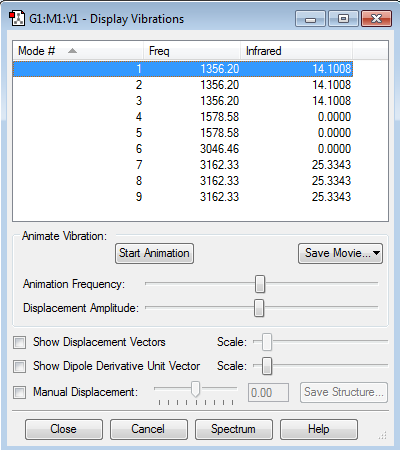
Vibration modes
• N2 has only one vibration mode which is bond stretch vibration. • No bands would appear in experimental spectrum.
Charge of atoms
• N2 has no charge on either of the atom as they are the same element and have same electronegativity.
H2
General information
| Molecule name | Calculation method | Basic set | Final energy E(RB3LYP) | RMS gradient | Point group | H-H bond distance | H-H structure |
|---|---|---|---|---|---|---|---|
| H2 | RB3LYP | 6-31G(d,p) | -1.17853936 a.u. | 0.00000017 a.u. | D*H | 0.74309Å | Linear |
Item Value Threshold Converged? Maximum Force 0.000211 0.000450 YES RMS Force 0.000211 0.000300 YES Maximum Displacement 0.000278 0.001800 YES RMS Displacement 0.000393 0.001200 YES
J mol of H2
test molecule |
Media:YONGYINCHEN_PHUNT_H2(2)_OPTF_POP.LOG
Vibration modes
• Similar to N2, H2 has only one vibration mode which is bond stretch vibration as well. • No bands would be seen in experimental spectrum.
Charge of atoms
• H2 has no charge on either of the atom as they are the same element and have same electronegativity.
The Haber-Bosch process
Equation
• N2 + 3H2 -> 2NH3
Energy of the reaction
• E(NH3)= -56.55776873 a.u. • 2*E(NH3)= -113.1155375 a.u. • E(N2)= -109.52412868 a.u. • E(H2)= -1.17853936 a.u. • 3*E(H2)= -3.53561808 a.u. • ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.0557907 a.u. = -146.48 kJ/mol • The energy of reaction is negative which indicates that this is an exothermic reaction and it actually is, therefore the product is more stable than the reactants.
But the value obtained is quite different from the literature experimental value (-92 kJ/mol)[1], because this value is calculated computationally.
CH4
General information
| Molecule name | Calculation method | Basic set | Final energy E(RB3LYP) | RMS gradient | Point group | C-H bond distance | H-C-H bond angle |
|---|---|---|---|---|---|---|---|
| CH4 | RB3LYP | 6-31G(d,p) | -40.44773109 a.u. | 0.04153842 a.u. | TD | 1.09194Å | 109.471 |
Item Value Threshold Converged? Maximum Force 0.000044 0.000450 YES RMS Force 0.000023 0.000300 YES Maximum Displacement 0.000124 0.001800 YES RMS Displacement 0.000066 0.001200 YES
J mol of CH4
test molecule |
Media:YONGYINCHEN_PHUNT_CH4_OPTF_POP.LOG
Vibration modes
• 9 modes of vibrations would be expected. • Modes 1&2&3 are degenerate to each other, so are modes 4&5 and modes 7&8&9. • 2 kinds of vibration modes are present. Modes 1,2,3,4,5 are bending vibrations and modes 6,7,8,9 are bond stretch vibrations. • Modes 4,5,6 is highly symmetric as the vibration of them do not produce dipole moment so they are IR inactive. • Therefore, 2 bands would be seen in the experimental spectrum of methane as expected.
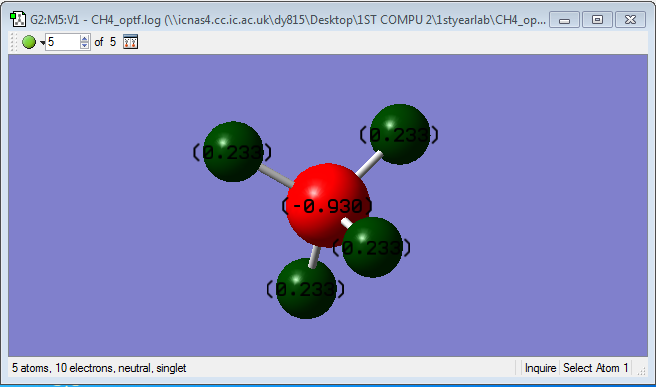
Charge of atoms
• C is more electronegative than H. Therefore, • C is expected to have a negative charge of -0.930 • H is expected to have a positive charge of +0.233
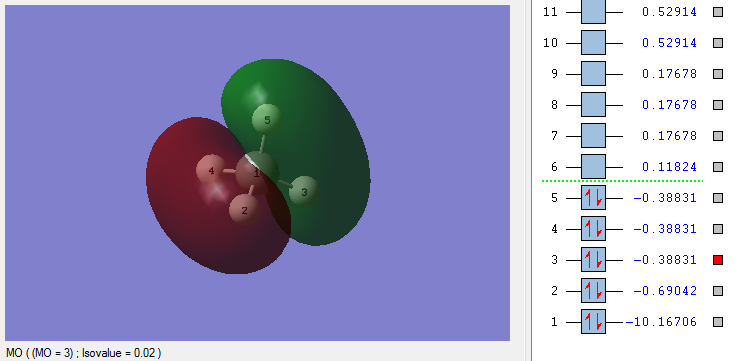
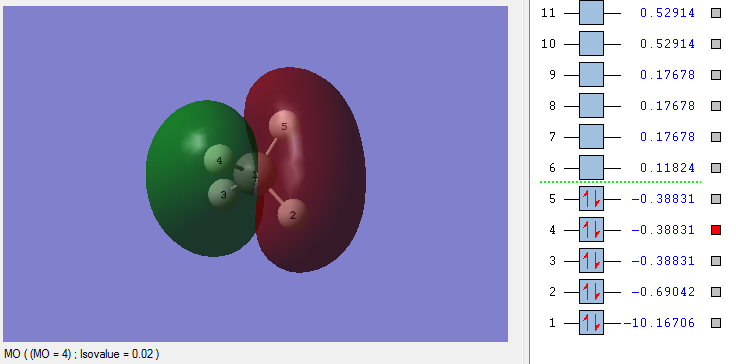
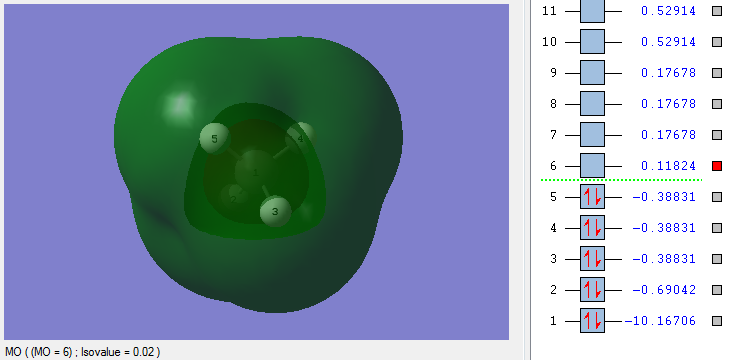
Molecular Orbitals (MO) of CH4
Reference
- ↑ http://www.chemguide.co.uk/physical/equilibria/haber.html (Accessed March 2018)