Rep:Mod:DS4113 TS
In this computational experiment, the thermodynamic properties, as well as the geometry and orbital considerations of the transition state, of 3 different pericyclic reactions has been calculated and compared to literature.
The reactions were:
- The Cope rearrangement of cyclohexa-1,5-diene
- The Diels-Alder reaction of ethene with butadiene
- The Diels-Alder reaction of maleic anhydride with cyclobutadiene.
The Cope Rearrangement
As an introduction into different computational methods used to calculate different aspects of two Diels-Alder reactions, the properties of Cope rearrangement of cyclohexa-1,5-diene were studied.

As a first step, two different geometries of cyclohexa-1,5-diene (one of them with 'gauge' geometry of the C2-C3-C4-C5 frame, the other one with an 'anti' geometry). The computations can result in a number of molecules with the stereochemical properties mentioned above ('gauge'/'anti'), but different overall symmetries. The first task was to find the lowest-energy conformation for both the ‘gauge’ and ‘anti’ system.
It was found that for ‘gauge’, the conformation of lowest energy had C1 symmetry (E = -234.61051914 a.u. // B3LYP / 6-31G). As for the ‚anti‘ geometry, the lowest-energy conformation had Ci symmetry and energy of E = -234.61171902 a.u. (B3LYP / 6-31G). As expected, the anti conformer is more stable compared to the gauge conformer. This observation can be simply explained by the fact that in the anti conformation, the steric clash of the remaining hydrocarbon chains is minimised. For the rearrangement to occur, the reacting molecule must have the right, gauge, geometry. The activation energy needed to change the conformation from the lowest -energy anti geometry to the needed gauge geometry is approximately between 1 to 2 kcal/mol, so it can be expected that even the RT, the change from one isomer to another should not be a major issue.
Nf710 (talk) 20:05, 21 January 2016 (UTC) You havent follwed the script here, you were meant to do it at 321G HF where the gauche is lower in energy, then you were meant to show why with the MOs, however i will give you some credit. although there is still alot of things you have missed out such as frequency calculations and thermochemistry. Follow the scrip next time.
The Chair and Boat TS

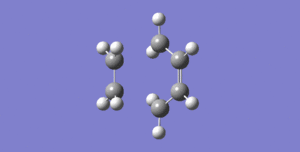
There are two possible transition state geometries for our our reaction; so-called ‘chair’ and ‘boat’ transition states. In general, the transition state of this reaction can be approximated using 2 allylic fragments, approximately 2.2 A apart. Why this is the case should be obvious from Fig. 1. It can be seen that at the transition state, new sigma-bond is partially formed, and the ‘opposite’ sigma-bond is partially broken. The same applies for the double bonds, the new two are partially formed, the original two double bonds are partially broken. This gives a structure resembling two allyl-like structures, partially bonded together. First, let’s look at the chair TS. As a first step, the approximate structure of the TS can be guessed and then optimised to TS (Berny). From literature, we know that the terminal ends of the allyls should be approximately 2.2 A apart. We also know that the symmetry of this TS is C2h, this can be used to symmetrise our initial guess to perhaps get even closer to the real transition state. The calculations then resulted in activation energy of the Cope Rearrangement going through the chair TS of 25.15 kcal/mol (B3LYP / 6-31G). The experimental activation energy is 33.5 kcal/mol. Considering that the transition state was merely a guess, the value obtained is relatively close, meaning that our initial guess could be close enough to the real TS structure (using a Hammond’s postulate - like argument). The optimised TS had inter-fragmet separation of 1.98 A, C1-C2-C3 angle of 120.17° (approx sp2 hybridised allyl atoms). The dihedral angle between C1-C2-C3-C3(of the second fragment) was found to be -65.67°.
Nf710 (talk) 20:09, 21 January 2016 (UTC) You should have got the correct energy here with B3LYP. so you have done something wrong.

Next, the properties of the boat TS were examined. This time using TS (QST2) method. In this method, two molecules are modelled. One of them is the starting material, the other is the product, QST2 then approximates between the two structures to find a transition state. Often, especially if the TS state is not clear from the structure of the molecules, it is better to change the structure of the molecules so that they somehow resemble the structure of the transition state, so it is more probable that when going from the reactants to the product, the algorithm ‘encounters’ the true T. Even if this attempt fails, the approximate structure of the transition state can be defined / guessed in the QST3 method to guide the algorithm through the transition state.
The activation energy for the proccess going through the boat TS was calculated to be 32.42 kcal/mol (B3LYP / 6-31G). For comparison, the calculated activation enthalpy was found to be 30.45 kcal/mol, the experimental value found in literature is 44.5 kcal/mol.[1] This can be due to poor guess of the transition state, that would result in lower energy. The point group symmetry for the optimised TS was C1, but under higher tolerance, it would symmetrise to C2 and with 0.01 tolerance to C2v.
Nf710 (talk) 20:13, 21 January 2016 (UTC) Again you should of got the correct answer.
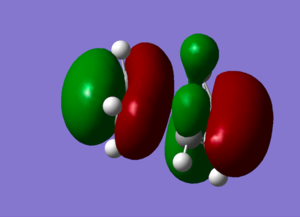
To ensure our results truly correspond to transition states, the vibrational frequencies of the expected transition-state structures was checked. For each, only one imaginary frequency was found (-520.21 for the boat TS, -568.18 for the chair TS), confirming that these structures really correspond to the TS as at the TS, there is one vibration mode correspospoding to the reaction coordinate. The curvature of the reaction coordinate is 'pointing downwards' (we are at the energy maximum), resulting in the force constant being negative, resulting in an imaginary frequency. If the imaginary vibrations are animated, it can be seen that the really correspond to the formation of new bonds /breaking of the old ones. (See Fig.2 .)
Nf710 (talk) 20:16, 21 January 2016 (UTC) good understanding of what a TS is. But you have missed out a lot of stuff so I cant really give you that many marks. perhaps you thought this part wasn't marked, but in future follow the script.
The Diels-Alder reaction
The Diels-Alder reaction of ethene and butadiene

The Diels-Alder reaction is a concerted reaction between a diene and a dienophile to form a cyclic system. The reaction was discovered in late 1920s by Otto Paul Herman Diels and Kurt Alder, for which they won the Nobel Prize in Chemistry in 1950. A class of so-called hetero-Diels-Alder reaction has been developed since as a one of the ways to syntesize cyclic systems containing hetero atoms.
(This diagram to the right seems incomplete. With no labels it's hard to see what's going on Tam10 (talk) 14:24, 11 January 2016 (UTC))
For most of the symmetric Diels-Alder reactions, the reaction mechanism is believed to go from the reactants to products in one, concerted, step. For substituted substrates, there seems to be a quite strong preference for specific stereospecificities, as opposed to 50:50 mixtures, as one could perhaps expect. This was explained in 1965 by R. B. Woodward and R. Hoffman in the famous ‘The Conservation of Orbital Symmetry’.
In 1952, Kenichi Fukui published (at that time a very controversial) paper on the idea that the reactions mechanisms are mainly governed by the HOMO/LUMO orbitals of the reacting molecules.[2] That speculation gave rise to a new method of studying chemical reactions called ‘Frontier molecular orbital theory’. Woodward and Hoffman than used this theory to explain then-not-very-understood pericyclic reactions, and came up with 4 rules. What’s most striking about their result is perhaps its simplicity...

In this part of the experiment, the two different Diels-Alder reactions were examined, and their thermodynamic properties , as well as the properties of the transition state, has been calculated. For most of the part of this experiment, the molecules were first optimised using 3-21g set, but the ultimate goal was to make results more accurate using the more B3LYP / 6-31G basis set. All the data presented in this part were calculated using the B3LYP / 6-31G method.
Frontier orbital interactions
The energy of the HOMO orbital can be approximated the ionization energy of a molecule; the energy of the LUMO orbital corresponds to electron affinity. It was found experimentally that the ionization energy of 1,3-butadiene is 9.07 eV and that of ethene is 10.51 eV. Electron affinities of 1,3-butadiene and ethene are -0.62 eV and -1.78 eV, respectively. For comparison, the energies of the HOMO orbitals were calculated (B3LYP / 6-31G basis set), the resulting HOMO energies were -6.16 eV and -7.26 eV for 1,3-butadiene and ethene, respectively. It is generally harder (less accurate) to obtain accurate energies of the LUMO orbitals by computational means. For example, calculations for LUMO of 1,3-butadiene resulted in a negative energy of the orbital. The energy of the LUMO orbital of ethene was calculated to be 0.51 eV. Based on these values, we were able construct the orbital interaction vs energy diagram shown on the left. The formation of only one new orbital is shown for simplicity.
For the reaction between s-cis-buta-1,3-diene and ethene, there is a flow of electrons from the HOMO of the diene to the LUMO of the dienophile (both have the right, a, symmetry). As a result of these electrons flowing to the LUMO orbital of the dienophile, the dienophile becomes more electron-rich (at the same time, the diene is becoming more electron-deficient), and a backwards flow of electron from the symmetric HOMO orbital of ethene to the symmetric LUMO orbital of the diene. This results in formation of 2 new sigma bonds. For visualization of different orbitals mentioned above, see Table 1.
| SLUMO Diene | 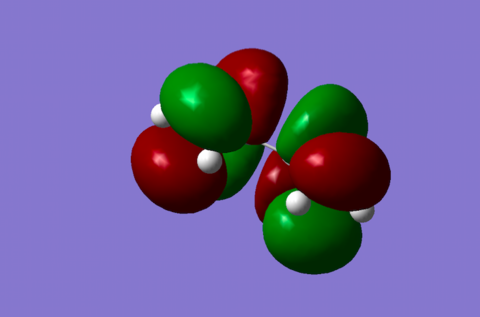
|
Asymmetric orbital with 3 nodes. This orbital rearranges into the LUMO orbital of the product.[3] |
| LUMO Diene | 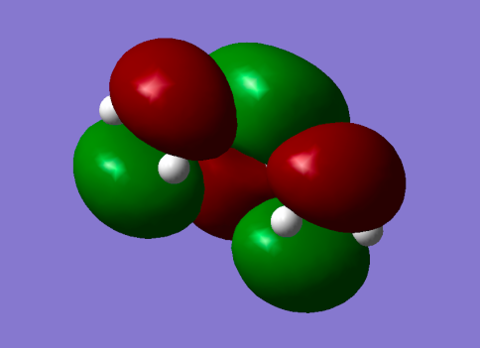
|
Symmetric orbital with 2 nodes. This orbital overlaps with the symmetric HOMO orbital of ethene during the reaction.[3] |
| HOMO Diene | 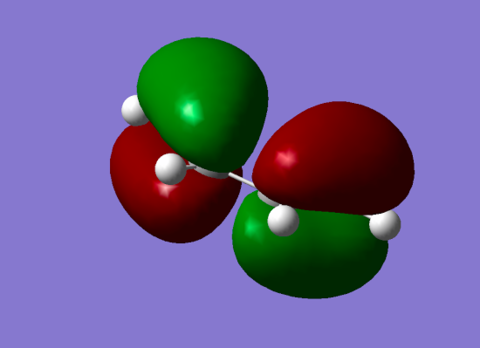
|
Antisymmetric orbital with 1 node. This orbital overlaps with the antisymmetric LUMO orbital of ethene during the reaction.[3] |
| NHOMO Diene | 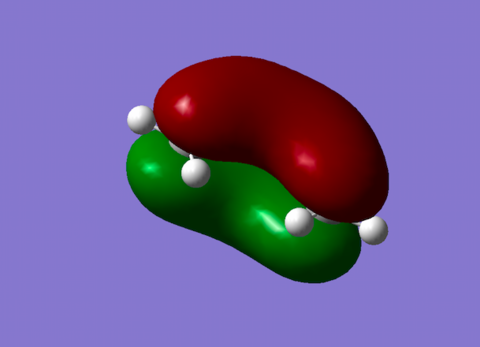
|
Symmetric orbital with no nodes. This orbital rearranges to become the HOMO orbital of the product.[3] |
| LUMO Dienophile | 
|
Asymmetric with 1 node. Reacts with the asymmetric HOMO orbital of the diene.[3] |
| HOMO Dienophile | 
|
Symmetric with 0 nodes. Reacts with the symmetric LUMO orbital of the diene.[3] |
Table 1. Frontier orbitals of the reacting molecules.
The transition state
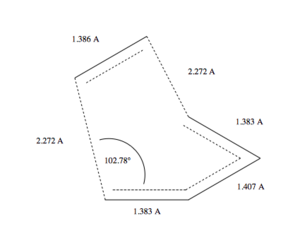
The geometry of the transition state structure can be seen bellow (Fig. 5). We can see that compared to 'standard', tabulated values of C-C bond lenghts, all the lenghts are almost the size of a triple bond! (This close packing of the atoms in the transition state could be one of the reasons behind the high energy of the TS.) That is because Gaussian uses a value of approx. 1.40 A for the length of the allyl bond (this value is actually true for benzene). Now that we know that the length of a bond of the order of 1.5 is 1.40 A, we can deduce that the ethene bond is still closer to double bond than a single bond. An interesting observation is that for the transition state, the lengths of the 'outer' bonds of the diene get shorter, and that of the middle bond gets larger. This can be justified by an increase of electron density on the ends of the molecule, and decrease of the electron density in the middle of the molecule, as electrons from the HOMO orbital now go into the LUMO orbital of the dienophile, resulting in lower conjugation in the TS. The value of the angle between the plane of ethene and the plane of the diene is 102.78°. This value fits our expectation, as it is in the region between 90° (sp2 coming to sp2 from above or bellow, as both the reactans are sp2 hybridized) and 109°, which corresponds to the sp3-sp3 bonding in the product. The angle between C1(ethene)-C2(ethene)-C1(diene)-C2(diene) was found to be -49.42°. All the data are in a excellent agreement in literature sources: inter-frangmet distance between 2.223 A and 2.240 A, middle C-C of the diene longer than other bonds, C1-C2-C1-C2 angle of -49.5°..
(A triple bond is perhaps a little over 1.2Å. Your later observations are more accurate though Tam10 (talk) 14:24, 11 January 2016 (UTC))
It is, however, true that the geometry optimisations result in geometries that are very similiar, no matter which set we use, so to compare the accuracy of our results, the following table contains a comparison between the ZPEs [kcal/mol] and thermal enthalpy corrections [kcal/mol] at 298.15 K using the B3LYP method and the 6-31G basis set:
| ZPE (calc) / [kcal/mol] | ZPE (lit) / [kcal/mol] | delta H corr (calc) / [kcal/mol] | delta H corr (lit) / [kcal/mol] | |
|---|---|---|---|---|
| cis-butadiene | 53.43 | 53.54 | 56.55 | 57.27 |
| ethene | 32.15 | 32.08 | 34.65 | 34.71 |
| cyclohexene | 92.20 | 91.95 | 96.24 | 96.28 |
| TS | 91.95 | 89.86 | 95.68 | 92.96 |
Table 2. Comparison of our results with literature.
We can see, that the values are in a great agreement with literature.
The data bellow (Table 3) suggest that the TS for this reaction is an early TS, as the bond length are more similar to the starting material. We can also mention that the new bonds are partially formed at the TS, as the distance between C(ethene)-C(butadiene) (2.27 A) as smaller than 2 Van der Waals radii of carbon (3.70 A).[4] Whether the transition state really is an early TS could be perhaps determined by comparing the enthalpies of the reactans with the enthalpy of the product. As is it expected that this reaction proceeds only through one TS, exothermal reaction would mean early-TS; endothermal reaction would hint at late TS.
The reaction enthalpy (at 298 K) was calculated to be -41.95 kcal/mol. We can, therefore, see that the reaction is exothermic. This is another hint the this reaction proceeds through an early-TS. The experimantal value stated in literature on computational chemistry was found to be approximately -50 kcal/mol, depending on the source. We ware . We were, however, unable to find the exact value.
The activation barrier for this eraction was calculated to be 34.87 kcal/mol, which is in range our computational results given calculated by Bach, McDouall and Schlegel[5]. The actual, experimental, value is 25.1 kcal/mol.[5]
| Reagents | TS | Product | |
|---|---|---|---|
| C-C of ethene | 1.31510 A | 1.38599 A | 1.53489 A |
| C1-C2 of butadiene | 1.33982 A | 1.38302 A | 1.50998 A |
| C2-C3 of butadiene | 1.471388 A | 1.40724 A | 1.33706 A |
| C(ethene) - C (butadiene) | - - - - - - - | 2.27232 A | 1.53739 A |

Table 3. Comparison of the bond lengths for reactants, TS and product.
Again, to make sure, the frequency analysis was run. It returned one imaginary frequency (-524.83)., so we can be sure we are at the TS. The animation of this 'vibration' can be seen in Fig.7. We can clearly see a synchronous bond formation. At the lowest positive frequency (135.78), the molecules vibrate in an asynchronous manner. (Fig. 8)
The reaction coordinate
Bellow, a 'hint' (only the structure of our concern are shown, not the whole reaction curve) of a reaction coordinate of the whole process (including cis/trans isomerisation of on of the starting materials) can be seen. (Fig. 6) We can see that the rate of isomerisation from the more stable trans-form to the Diels-Alder active cis-form should not affect the overall rate of the reaction, as the activation barrier is much smaller, compared to the energy barrier for the Diels-Alder reaction. We can also see that under thermodynamic conditions, the reaction should proceed to almost completion as the energy of the products is much lower than that of the reactants. (N.B. The energy of the A+s-cis-B system was chosen to be zero, so that, if needed, any population analysis would be easy to be done compared the 'stereochemically-right' starting material.
(This is good. As you say, it would be very difficult to produce the actual reaction coordinate - finding TS1 with A would be the trickiest part Tam10 (talk) 14:24, 11 January 2016 (UTC))

Cyclohexadiene and maleic anhydride

As a general rule, it was found experimentally that at lower T (thermodynamic control), a so-called endo-product is favourably formed, whereas under thermodynamic conditions the opposite is true. From this fact, we can deduce that the TS leading to the endo-product should be lower in energy (reason behind this is probably secondary overlap of accumulated pi-bonds). From the fact that under thermodynamic conditions the exo-product is favoured, we can deduce that it should be lower in energy compared to the endo-product. (less steric strain in the endo-product)[6]. (Situation depicted in Fig. 9.)
In this, final part, of this experiment, we will try to determine computationally whether the above deductions are true even for our compound of interest.
First, the energy of the reacting molecules was calculated, then those of the product. What is interesting is that during the calculations for the endo-product, the endo-product spontaneously flipped into the exo-form. This, by itself, could be used as a prove that the exo-product is lower in energy. The calculation was then run again, this time using a somewhat restricted geometry to make sure the flip will not occur again, but every time resulted in error. The same, for some reason, happened even to the endo TS, even thought all the methods mentioned in the script (Berny, frozen coordinates, QST2, QST3,..) have been tried. All the computation were done using the 6-31G basis set. The thermochemical properties could be therefore only calculated for the exo-reaction. Enough literature data was, however, found to fill in for the endo-reaction.
(How did the endo product spontaneously flip to the exo product? Tam10 (talk) 14:24, 11 January 2016 (UTC))
Using the same logic as above, the reaction enthalpy going to the exo product was found to be -27.29 kcal/mol. The activation energy for the reaction of maleic anhydride with cyclopentadiene stated in literature is -17.51 kcal/mol.[7] This could be due to lower stability of the five-membered bicyclic compound, as opposed to our six-membered bicyclic product. The activation barrier was found to be 14.96 kcal/mol. [7]
As for the endo-product, the reaction enthaphy was found to be -16.74 kcal/mol. [7] The activation barrier of this reaction is 13.95 kcal/mol. We can see that our predictions at the beginning of the paragraph were actually correct.
(These values seem a little off. You haven't put in your calculations, log files or any jmols so I can't tell what's happened. Tam10 (talk) 14:24, 11 January 2016 (UTC))
Secondary interactions
As proposed by Woodward and Hoffman, the lower activation barrier for the formation of the endo product could be caused by a secondary interaction between the C=O and C=C double bonds accumulated in the region. Another explanation, given by Herndon and Hall, is that the lower energy of the endo-TS results from a more efficient orbital overlap in this geometry. This assumption was based on calculations of overlap orbitals. Herdon's and Hall's theory is somewhat more general as it can be used even for systems where there is no secondary orbital interaction.[6]
Conclussion
The properties of a variety of different chemical systems undergoing pericyclic reactions were computationally calculated. The transition state geometries, as well as the TS molecular orbitals were also calculated and presented. For the calculation, we used an, now widely accepted, assumption that the reactions proceed in concerted fashion through one and only transition state. There is still, however, some ambiguity, as some experiment suggests that, at least in some cases, the pericyclic reactions could actually proceed by a stepwise mechanism (through an intermediate diradical, for example). This should be a consideration when trying to calculate even more accurate data.
One of the flaws of computational methods is that they over- or under- estimate the real value, so the data obtained computationally must then usually be multiplied by a coefficient to get as accurate data as possible. This hasn't been done for this report, as there was simply not enough data to construct to correction from.
Most of the analysis was shown on the ethene + butadiene, because not enough data was gathered for the endo-reaction pathway of the last part. Should repeat the experiment again, careful investigation would be taken to find the source of the error, that repeatedly caused the calculations to fail, to ensure we obtain the optimised structures of the endo-TS and endo-product.
Equations used
and similarly:
Thermochemical Raw data
Gauge
-------------------
- Thermochemistry -
-------------------
Temperature 298.150 Kelvin. Pressure 1.00000 Atm.
Atom 1 has atomic number 6 and mass 12.00000
Atom 2 has atomic number 1 and mass 1.00783
Atom 3 has atomic number 1 and mass 1.00783
Atom 4 has atomic number 6 and mass 12.00000
Atom 5 has atomic number 1 and mass 1.00783
Atom 6 has atomic number 6 and mass 12.00000
Atom 7 has atomic number 1 and mass 1.00783
Atom 8 has atomic number 1 and mass 1.00783
Atom 9 has atomic number 6 and mass 12.00000
Atom 10 has atomic number 1 and mass 1.00783
Atom 11 has atomic number 1 and mass 1.00783
Atom 12 has atomic number 6 and mass 12.00000
Atom 13 has atomic number 1 and mass 1.00783
Atom 14 has atomic number 6 and mass 12.00000
Atom 15 has atomic number 1 and mass 1.00783
Atom 16 has atomic number 1 and mass 1.00783
Molecular mass: 82.07825 amu.
Principal axes and moments of inertia in atomic units:
1 2 3
Eigenvalues -- 176.041071129.992601207.95741
X 0.99997 0.00000 0.00797
Y 0.00000 1.00000 0.00000
Z -0.00797 0.00000 0.99997
This molecule is an asymmetric top.
Rotational symmetry number 1.
Rotational temperatures (Kelvin) 0.49201 0.07665 0.07170
Rotational constants (GHZ): 10.25182 1.59713 1.49404
Zero-point vibrational energy 374720.4 (Joules/Mol)
89.56032 (Kcal/Mol)
Warning -- explicit consideration of 7 degrees of freedom as
vibrations may cause significant error
Vibrational temperatures: 94.83 100.10 204.80 359.31 518.69
(Kelvin) 587.74 644.73 907.32 1019.29 1176.65
1218.19 1353.06 1353.62 1366.22 1440.65
1474.91 1490.42 1509.26 1634.57 1648.91
1790.34 1861.89 1913.55 1924.63 1959.63
1985.01 2118.91 2129.57 2176.52 2181.56
2490.38 2492.44 4354.58 4359.97 4425.52
4430.39 4520.77 4531.31 4539.92 4542.84
4651.71 4652.18
Zero-point correction= 0.142723 (Hartree/Particle)
Thermal correction to Energy= 0.150003
Thermal correction to Enthalpy= 0.150947
Thermal correction to Gibbs Free Energy= 0.111053
Sum of electronic and zero-point Energies= -234.467796
Sum of electronic and thermal Energies= -234.460516
Sum of electronic and thermal Enthalpies= -234.459572
Sum of electronic and thermal Free Energies= -234.499466
E (Thermal) CV S
KCal/Mol Cal/Mol-Kelvin Cal/Mol-Kelvin
Total 94.128 25.339 83.963
Electronic 0.000 0.000 0.000
Translational 0.889 2.981 39.129
Rotational 0.889 2.981 26.977
Vibrational 92.351 19.377 17.857
Vibration 1 0.597 1.971 4.272
Vibration 2 0.598 1.969 4.165
Vibration 3 0.616 1.911 2.772
Vibration 4 0.663 1.763 1.732
Vibration 5 0.735 1.554 1.120
Vibration 6 0.773 1.452 0.932
Vibration 7 0.807 1.365 0.802
Q Log10(Q) Ln(Q)
Total Bot 0.829995D-51 -51.080925 -117.618176
Total V=0 0.369119D+15 14.567166 33.542140
Vib (Bot) 0.161831D-63 -63.790939 -146.884065
Vib (Bot) 1 0.313067D+01 0.495637 1.141247
Vib (Bot) 2 0.296454D+01 0.471957 1.086722
Vib (Bot) 3 0.142755D+01 0.154591 0.355958
Vib (Bot) 4 0.781619D+00 -0.107005 -0.246388
Vib (Bot) 5 0.508246D+00 -0.293926 -0.676790
Vib (Bot) 6 0.433587D+00 -0.362923 -0.835662
Vib (Bot) 7 0.383279D+00 -0.416485 -0.958993
Vib (V=0) 0.719701D+02 1.857152 4.276251
Vib (V=0) 1 0.367035D+01 0.564707 1.300286
Vib (V=0) 2 0.350641D+01 0.544863 1.254593
Vib (V=0) 3 0.201258D+01 0.303753 0.699417
Vib (V=0) 4 0.142786D+01 0.154686 0.356178
Vib (V=0) 5 0.121296D+01 0.083847 0.193065
Vib (V=0) 6 0.116181D+01 0.065137 0.149983
Vib (V=0) 7 0.113000D+01 0.053079 0.122220
Electronic 0.100000D+01 0.000000 0.000000
Translational 0.292279D+08 7.465797 17.190634
Rotational 0.175476D+06 5.244217 12.075256
Anti
-------------------
- Thermochemistry -
-------------------
Temperature 298.150 Kelvin. Pressure 1.00000 Atm.
Atom 1 has atomic number 6 and mass 12.00000
Atom 2 has atomic number 6 and mass 12.00000
Atom 3 has atomic number 6 and mass 12.00000
Atom 4 has atomic number 6 and mass 12.00000
Atom 5 has atomic number 6 and mass 12.00000
Atom 6 has atomic number 6 and mass 12.00000
Atom 7 has atomic number 1 and mass 1.00783
Atom 8 has atomic number 1 and mass 1.00783
Atom 9 has atomic number 1 and mass 1.00783
Atom 10 has atomic number 1 and mass 1.00783
Atom 11 has atomic number 1 and mass 1.00783
Atom 12 has atomic number 1 and mass 1.00783
Atom 13 has atomic number 1 and mass 1.00783
Atom 14 has atomic number 1 and mass 1.00783
Atom 15 has atomic number 1 and mass 1.00783
Atom 16 has atomic number 1 and mass 1.00783
Molecular mass: 82.07825 amu.
Principal axes and moments of inertia in atomic units:
1 2 3
Eigenvalues -- 406.00527 509.82816 794.94212
X 0.00000 0.43551 0.90019
Y 0.00000 0.90019 -0.43551
Z 1.00000 0.00000 0.00000
This molecule is an asymmetric top.
Rotational symmetry number 1.
Rotational temperatures (Kelvin) 0.21333 0.16989 0.10896
Rotational constants (GHZ): 4.44512 3.53990 2.27028
1 imaginary frequencies ignored.
Zero-point vibrational energy 368282.4 (Joules/Mol)
88.02160 (Kcal/Mol)
Warning -- explicit consideration of 7 degrees of freedom as
vibrations may cause significant error
Vibrational temperatures: 192.23 368.67 481.80 544.42 570.86
(Kelvin) 583.17 626.17 1067.01 1098.15 1117.07
1187.40 1231.13 1372.76 1399.81 1412.88
1446.09 1451.54 1485.77 1492.14 1543.48
1546.62 1838.16 1843.36 1852.48 1867.18
2068.31 2083.68 2205.22 2224.08 2256.63
2352.70 4509.01 4513.77 4524.67 4530.72
4536.81 4545.20 4644.69 4646.51 4660.27
4665.65
Zero-point correction= 0.140271 (Hartree/Particle)
Thermal correction to Energy= 0.146654
Thermal correction to Enthalpy= 0.147599
Thermal correction to Gibbs Free Energy= 0.110816
Sum of electronic and zero-point Energies= -234.418365
Sum of electronic and thermal Energies= -234.411982
Sum of electronic and thermal Enthalpies= -234.411037
Sum of electronic and thermal Free Energies= -234.447820
E (Thermal) CV S
KCal/Mol Cal/Mol-Kelvin Cal/Mol-Kelvin
Total 92.027 24.715 77.415
Electronic 0.000 0.000 0.000
Translational 0.889 2.981 39.129
Rotational 0.889 2.981 26.601
Vibrational 90.250 18.754 11.686
Vibration 1 0.613 1.920 2.893
Vibration 2 0.666 1.752 1.687
Vibration 3 0.716 1.606 1.236
Vibration 4 0.749 1.516 1.046
Vibration 5 0.763 1.477 0.975
Vibration 6 0.770 1.459 0.943
Vibration 7 0.796 1.393 0.842
Q Log10(Q) Ln(Q)
Total Bot 0.106723D-50 -50.971742 -117.366774
Total V=0 0.353558D+14 13.548461 31.196484
Vib (Bot) 0.251460D-63 -63.599532 -146.443333
Vib (Bot) 1 0.152443D+01 0.183107 0.421620
Vib (Bot) 2 0.759393D+00 -0.119533 -0.275236
Vib (Bot) 3 0.556287D+00 -0.254701 -0.586471
Vib (Bot) 4 0.478365D+00 -0.320241 -0.737382
Vib (Bot) 5 0.450279D+00 -0.346518 -0.797888
Vib (Bot) 6 0.438021D+00 -0.358505 -0.825489
Vib (Bot) 7 0.398720D+00 -0.399332 -0.919495
Vib (V=0) 0.833051D+01 0.920672 2.119925
Vib (V=0) 1 0.210433D+01 0.323114 0.743999
Vib (V=0) 2 0.140922D+01 0.148978 0.343035
Vib (V=0) 3 0.124797D+01 0.096203 0.221516
Vib (V=0) 4 0.119198D+01 0.076268 0.175614
Vib (V=0) 5 0.117287D+01 0.069249 0.159452
Vib (V=0) 6 0.116473D+01 0.066224 0.152487
Vib (V=0) 7 0.113951D+01 0.056720 0.130602
Electronic 0.100000D+01 0.000000 0.000000
Translational 0.292279D+08 7.465797 17.190634
Rotational 0.145208D+06 5.161992 11.885926
Chair TS
-------------------
- Thermochemistry -
-------------------
Temperature 298.150 Kelvin. Pressure 1.00000 Atm.
Atom 1 has atomic number 6 and mass 12.00000
Atom 2 has atomic number 1 and mass 1.00783
Atom 3 has atomic number 6 and mass 12.00000
Atom 4 has atomic number 1 and mass 1.00783
Atom 5 has atomic number 1 and mass 1.00783
Atom 6 has atomic number 6 and mass 12.00000
Atom 7 has atomic number 1 and mass 1.00783
Atom 8 has atomic number 1 and mass 1.00783
Atom 9 has atomic number 6 and mass 12.00000
Atom 10 has atomic number 1 and mass 1.00783
Atom 11 has atomic number 6 and mass 12.00000
Atom 12 has atomic number 1 and mass 1.00783
Atom 13 has atomic number 1 and mass 1.00783
Atom 14 has atomic number 6 and mass 12.00000
Atom 15 has atomic number 1 and mass 1.00783
Atom 16 has atomic number 1 and mass 1.00783
Molecular mass: 82.07825 amu.
Principal axes and moments of inertia in atomic units:
1 2 3
Eigenvalues -- 399.59797 447.34112 737.43711
X 0.99989 0.00405 0.01401
Y -0.00405 0.99999 -0.00010
Z -0.01401 0.00004 0.99990
This molecule is an asymmetric top.
Rotational symmetry number 1.
Rotational temperatures (Kelvin) 0.21675 0.19362 0.11745
Rotational constants (GHZ): 4.51639 4.03437 2.44732
1 imaginary frequencies ignored.
Zero-point vibrational energy 371669.1 (Joules/Mol)
88.83104 (Kcal/Mol)
Warning -- explicit consideration of 7 degrees of freedom as
vibrations may cause significant error
Vibrational temperatures: 277.38 373.79 541.96 552.72 632.80
(Kelvin) 695.54 739.66 1119.11 1135.29 1183.40
1256.86 1341.18 1419.60 1421.22 1427.76
1486.62 1493.92 1503.32 1598.13 1603.46
1646.65 1804.88 1820.08 1858.08 1862.79
2057.92 2100.64 2212.33 2213.30 2227.08
2311.87 4497.40 4498.89 4502.79 4504.03
4522.48 4523.88 4603.99 4608.39 4609.50
4612.23
Zero-point correction= 0.141561 (Hartree/Particle)
Thermal correction to Energy= 0.147522
Thermal correction to Enthalpy= 0.148466
Thermal correction to Gibbs Free Energy= 0.112640
Sum of electronic and zero-point Energies= -234.430453
Sum of electronic and thermal Energies= -234.424492
Sum of electronic and thermal Enthalpies= -234.423548
Sum of electronic and thermal Free Energies= -234.459374
E (Thermal) CV S
KCal/Mol Cal/Mol-Kelvin Cal/Mol-Kelvin
Total 92.572 23.467 75.402
Electronic 0.000 0.000 0.000
Translational 0.889 2.981 39.129
Rotational 0.889 2.981 26.380
Vibrational 90.794 17.505 9.893
Vibration 1 0.635 1.850 2.201
Vibration 2 0.668 1.746 1.663
Vibration 3 0.747 1.520 1.052
Vibration 4 0.753 1.504 1.023
Vibration 5 0.800 1.383 0.827
Vibration 6 0.840 1.287 0.701
Vibration 7 0.869 1.219 0.624
Q Log10(Q) Ln(Q)
Total Bot 0.154589D-51 -51.810820 -119.298823
Total V=0 0.200774D+14 13.302708 30.630616
Vib (Bot) 0.406951D-64 -64.390458 -148.264508
Vib (Bot) 1 0.103708D+01 0.015811 0.036406
Vib (Bot) 2 0.747697D+00 -0.126275 -0.290758
Vib (Bot) 3 0.481110D+00 -0.317756 -0.731660
Vib (Bot) 4 0.469283D+00 -0.328565 -0.756549
Vib (Bot) 5 0.393110D+00 -0.405486 -0.933667
Vib (Bot) 6 0.344941D+00 -0.462256 -1.064383
Vib (Bot) 7 0.315678D+00 -0.500755 -1.153031
Vib (V=0) 0.528531D+01 0.723070 1.664931
Vib (V=0) 1 0.165132D+01 0.217830 0.501573
Vib (V=0) 2 0.139947D+01 0.145964 0.336095
Vib (V=0) 3 0.119388D+01 0.076960 0.177207
Vib (V=0) 4 0.118573D+01 0.073986 0.170359
Vib (V=0) 5 0.113603D+01 0.055390 0.127540
Vib (V=0) 6 0.110744D+01 0.044320 0.102052
Vib (V=0) 7 0.109131D+01 0.037950 0.087383
Electronic 0.100000D+01 0.000000 0.000000
Translational 0.292279D+08 7.465797 17.190634
Rotational 0.129969D+06 5.113840 11.775052
Boat TS
-------------------
- Thermochemistry -
-------------------
Temperature 298.150 Kelvin. Pressure 1.00000 Atm.
Atom 1 has atomic number 6 and mass 12.00000
Atom 2 has atomic number 6 and mass 12.00000
Atom 3 has atomic number 6 and mass 12.00000
Atom 4 has atomic number 6 and mass 12.00000
Atom 5 has atomic number 6 and mass 12.00000
Atom 6 has atomic number 6 and mass 12.00000
Atom 7 has atomic number 1 and mass 1.00783
Atom 8 has atomic number 1 and mass 1.00783
Atom 9 has atomic number 1 and mass 1.00783
Atom 10 has atomic number 1 and mass 1.00783
Atom 11 has atomic number 1 and mass 1.00783
Atom 12 has atomic number 1 and mass 1.00783
Atom 13 has atomic number 1 and mass 1.00783
Atom 14 has atomic number 1 and mass 1.00783
Atom 15 has atomic number 1 and mass 1.00783
Atom 16 has atomic number 1 and mass 1.00783
Molecular mass: 82.07825 amu.
Principal axes and moments of inertia in atomic units:
1 2 3
Eigenvalues -- 406.04878 509.90313 795.15681
X 1.00000 -0.00008 0.00002
Y 0.00008 1.00000 0.00000
Z -0.00002 0.00000 1.00000
This molecule is an asymmetric top.
Rotational symmetry number 1.
Rotational temperatures (Kelvin) 0.21331 0.16986 0.10893
Rotational constants (GHZ): 4.44464 3.53938 2.26967
1 imaginary frequencies ignored.
Zero-point vibrational energy 368335.4 (Joules/Mol)
88.03427 (Kcal/Mol)
Warning -- explicit consideration of 7 degrees of freedom as
vibrations may cause significant error
Vibrational temperatures: 194.34 373.35 482.90 547.40 577.20
(Kelvin) 579.62 626.19 1068.36 1101.15 1117.12
1185.74 1231.93 1370.38 1400.32 1409.82
1446.66 1451.28 1486.61 1493.47 1543.51
1546.54 1838.31 1842.60 1852.01 1868.38
2068.56 2084.01 2203.72 2223.41 2256.10
2352.90 4509.53 4514.40 4524.43 4530.28
4536.94 4545.12 4644.63 4646.16 4660.20
4665.46
Zero-point correction= 0.140292 (Hartree/Particle)
Thermal correction to Energy= 0.146662
Thermal correction to Enthalpy= 0.147606
Thermal correction to Gibbs Free Energy= 0.110854
Sum of electronic and zero-point Energies= -234.418363
Sum of electronic and thermal Energies= -234.411993
Sum of electronic and thermal Enthalpies= -234.411048
Sum of electronic and thermal Free Energies= -234.447801
E (Thermal) CV S
KCal/Mol Cal/Mol-Kelvin Cal/Mol-Kelvin
Total 92.032 24.696 77.352
Electronic 0.000 0.000 0.000
Translational 0.889 2.981 39.129
Rotational 0.889 2.981 26.601
Vibrational 90.254 18.735 11.622
Vibration 1 0.613 1.918 2.872
Vibration 2 0.668 1.747 1.665
Vibration 3 0.717 1.604 1.233
Vibration 4 0.750 1.512 1.037
Vibration 5 0.767 1.468 0.958
Vibration 6 0.768 1.464 0.952
Vibration 7 0.796 1.393 0.842
Q Log10(Q) Ln(Q)
Total Bot 0.102544D-50 -50.989089 -117.406716
Total V=0 0.347059D+14 13.540403 31.177931
Vib (Bot) 0.241551D-63 -63.616992 -146.483537
Vib (Bot) 1 0.150732D+01 0.178204 0.410330
Vib (Bot) 2 0.748686D+00 -0.125700 -0.289436
Vib (Bot) 3 0.554761D+00 -0.255894 -0.589218
Vib (Bot) 4 0.475073D+00 -0.323240 -0.744287
Vib (Bot) 5 0.443899D+00 -0.352715 -0.812157
Vib (Bot) 6 0.441505D+00 -0.355065 -0.817566
Vib (Bot) 7 0.398708D+00 -0.399345 -0.919526
Vib (V=0) 0.817523D+01 0.912500 2.101109
Vib (V=0) 1 0.208808D+01 0.319747 0.736245
Vib (V=0) 2 0.140029D+01 0.146219 0.336683
Vib (V=0) 3 0.124683D+01 0.095808 0.220607
Vib (V=0) 4 0.118971D+01 0.075440 0.173706
Vib (V=0) 5 0.116862D+01 0.067672 0.155820
Vib (V=0) 6 0.116703D+01 0.067081 0.154460
Vib (V=0) 7 0.113951D+01 0.056717 0.130595
Electronic 0.100000D+01 0.000000 0.000000
Translational 0.292279D+08 7.465797 17.190634
Rotational 0.145247D+06 5.162106 11.886188
trans-butadiene
-------------------
- Thermochemistry -
-------------------
Temperature 298.150 Kelvin. Pressure 1.00000 Atm.
Atom 1 has atomic number 6 and mass 12.00000
Atom 2 has atomic number 1 and mass 1.00783
Atom 3 has atomic number 1 and mass 1.00783
Atom 4 has atomic number 6 and mass 12.00000
Atom 5 has atomic number 1 and mass 1.00783
Atom 6 has atomic number 6 and mass 12.00000
Atom 7 has atomic number 1 and mass 1.00783
Atom 8 has atomic number 6 and mass 12.00000
Atom 9 has atomic number 1 and mass 1.00783
Atom 10 has atomic number 1 and mass 1.00783
Molecular mass: 54.04695 amu.
Principal axes and moments of inertia in atomic units:
1 2 3
Eigenvalues -- 42.62552 409.01495 451.64047
X -0.54545 0.83814 0.00000
Y 0.83814 0.54545 0.00000
Z 0.00000 0.00000 1.00000
This molecule is an asymmetric top.
Rotational symmetry number 1.
Rotational temperatures (Kelvin) 2.03197 0.21176 0.19178
Rotational constants (GHZ): 42.33945 4.41241 3.99597
Zero-point vibrational energy 223969.3 (Joules/Mol)
53.52995 (Kcal/Mol)
Warning -- explicit consideration of 4 degrees of freedom as
vibrations may cause significant error
Vibrational temperatures: 254.62 425.88 742.91 776.99 1124.68
(Kelvin) 1301.82 1338.83 1344.05 1443.27 1447.27
1530.17 1776.16 1902.85 1904.49 2051.93
2137.77 2407.41 2484.14 4521.09 4535.32
4542.43 4543.48 4668.22 4668.79
Zero-point correction= 0.085305 (Hartree/Particle)
Thermal correction to Energy= 0.089954
Thermal correction to Enthalpy= 0.090898
Thermal correction to Gibbs Free Energy= 0.058859
Sum of electronic and zero-point Energies= -155.916358
Sum of electronic and thermal Energies= -155.911709
Sum of electronic and thermal Enthalpies= -155.910765
Sum of electronic and thermal Free Energies= -155.942804
E (Thermal) CV S
KCal/Mol Cal/Mol-Kelvin Cal/Mol-Kelvin
Total 56.447 15.743 67.433
Electronic 0.000 0.000 0.000
Translational 0.889 2.981 37.884
Rotational 0.889 2.981 23.580
Vibrational 54.670 9.781 5.969
Vibration 1 0.628 1.871 2.360
Vibration 2 0.690 1.681 1.439
Vibration 3 0.871 1.214 0.618
Vibration 4 0.895 1.162 0.565
Q Log10(Q) Ln(Q)
Total Bot 0.845235D-27 -27.073022 -62.337938
Total V=0 0.146103D+13 12.164658 28.010161
Vib (Bot) 0.170380D-38 -38.768582 -89.267958
Vib (Bot) 1 0.113611D+01 0.055422 0.127614
Vib (Bot) 2 0.643927D+00 -0.191163 -0.440170
Vib (Bot) 3 0.313652D+00 -0.503552 -1.159472
Vib (Bot) 4 0.293370D+00 -0.532584 -1.226319
Vib (V=0) 0.294509D+01 0.469099 1.080141
Vib (V=0) 1 0.174127D+01 0.240867 0.554616
Vib (V=0) 2 0.131526D+01 0.119010 0.274031
Vib (V=0) 3 0.109024D+01 0.037520 0.086393
Vib (V=0) 4 0.107971D+01 0.033308 0.076694
Electronic 0.100000D+01 0.000000 0.000000
Translational 0.156175D+08 7.193612 16.563904
Rotational 0.317649D+05 4.501947 10.366117
cis-butadiene
-------------------
- Thermochemistry -
-------------------
Temperature 298.150 Kelvin. Pressure 1.00000 Atm.
Atom 1 has atomic number 6 and mass 12.00000
Atom 2 has atomic number 1 and mass 1.00783
Atom 3 has atomic number 6 and mass 12.00000
Atom 4 has atomic number 1 and mass 1.00783
Atom 5 has atomic number 1 and mass 1.00783
Atom 6 has atomic number 6 and mass 12.00000
Atom 7 has atomic number 1 and mass 1.00783
Atom 8 has atomic number 6 and mass 12.00000
Atom 9 has atomic number 1 and mass 1.00783
Atom 10 has atomic number 1 and mass 1.00783
Molecular mass: 54.04695 amu.
Principal axes and moments of inertia in atomic units:
1 2 3
Eigenvalues -- 83.94112 321.37815 405.31927
X -0.60772 0.79415 0.00000
Y 0.79415 0.60772 0.00000
Z 0.00000 0.00000 1.00000
This molecule is an asymmetric top.
Rotational symmetry number 1.
Rotational temperatures (Kelvin) 1.03184 0.26951 0.21369
Rotational constants (GHZ): 21.50008 5.61563 4.45264
1 imaginary frequencies ignored.
Zero-point vibrational energy 223539.4 (Joules/Mol)
53.42719 (Kcal/Mol)
Warning -- explicit consideration of 3 degrees of freedom as
vibrations may cause significant error
Vibrational temperatures: 427.25 745.30 819.20 1076.91 1279.10
(Kelvin) 1334.82 1338.10 1493.86 1496.95 1545.50
1614.53 1915.68 1977.93 2103.70 2143.09
2433.56 2481.78 4530.11 4550.41 4555.34
4565.62 4668.78 4673.63
Zero-point correction= 0.085142 (Hartree/Particle)
Thermal correction to Energy= 0.089181
Thermal correction to Enthalpy= 0.090126
Thermal correction to Gibbs Free Energy= 0.059072
Sum of electronic and zero-point Energies= -155.900814
Sum of electronic and thermal Energies= -155.896775
Sum of electronic and thermal Enthalpies= -155.895831
Sum of electronic and thermal Free Energies= -155.926884
E (Thermal) CV S
KCal/Mol Cal/Mol-Kelvin Cal/Mol-Kelvin
Total 55.962 13.847 65.358
Electronic 0.000 0.000 0.000
Translational 0.889 2.981 37.884
Rotational 0.889 2.981 23.907
Vibrational 54.185 7.885 3.568
Vibration 1 0.691 1.679 1.434
Vibration 2 0.873 1.210 0.615
Vibration 3 0.925 1.098 0.505
Q Log10(Q) Ln(Q)
Total Bot 0.674477D-27 -27.171033 -62.563615
Total V=0 0.980224D+12 11.991326 27.611047
Vib (Bot) 0.115376D-38 -38.937885 -89.657795
Vib (Bot) 1 0.641518D+00 -0.192791 -0.443918
Vib (Bot) 2 0.312169D+00 -0.505610 -1.164210
Vib (Bot) 3 0.270474D+00 -0.567874 -1.307578
Vib (V=0) 0.167677D+01 0.224473 0.516868
Vib (V=0) 1 0.131335D+01 0.118382 0.272585
Vib (V=0) 2 0.108945D+01 0.037207 0.085672
Vib (V=0) 3 0.106847D+01 0.028762 0.066226
Electronic 0.100000D+01 0.000000 0.000000
Translational 0.156175D+08 7.193612 16.563904
Rotational 0.374318D+05 4.573241 10.530276
Ethene
-------------------
- Thermochemistry -
-------------------
Temperature 298.150 Kelvin. Pressure 1.00000 Atm.
Atom 1 has atomic number 6 and mass 12.00000
Atom 2 has atomic number 1 and mass 1.00783
Atom 3 has atomic number 1 and mass 1.00783
Atom 4 has atomic number 6 and mass 12.00000
Atom 5 has atomic number 1 and mass 1.00783
Atom 6 has atomic number 1 and mass 1.00783
Molecular mass: 28.03130 amu.
Principal axes and moments of inertia in atomic units:
1 2 3
Eigenvalues -- 12.27771 60.07760 72.35530
X 0.00000 1.00000 0.00000
Y 1.00000 0.00000 0.00000
Z 0.00000 0.00000 1.00000
This molecule is an asymmetric top.
Rotational symmetry number 2.
Rotational temperatures (Kelvin) 7.05456 1.44170 1.19706
Rotational constants (GHZ): 146.99336 30.04017 24.94276
Zero-point vibrational energy 134497.9 (Joules/Mol)
32.14578 (Kcal/Mol)
Vibrational temperatures: 1201.54 1375.83 1404.24 1539.33 1795.51
(Kelvin) 2008.53 2150.51 2475.66 4535.16 4557.41
4636.13 4672.87
Zero-point correction= 0.051228 (Hartree/Particle)
Thermal correction to Energy= 0.054269
Thermal correction to Enthalpy= 0.055214
Thermal correction to Gibbs Free Energy= 0.029698
Sum of electronic and zero-point Energies= -78.536231
Sum of electronic and thermal Energies= -78.533189
Sum of electronic and thermal Enthalpies= -78.532245
Sum of electronic and thermal Free Energies= -78.557760
E (Thermal) CV S
KCal/Mol Cal/Mol-Kelvin Cal/Mol-Kelvin
Total 34.055 8.088 53.702
Electronic 0.000 0.000 0.000
Translational 0.889 2.981 35.927
Rotational 0.889 2.981 17.241
Vibrational 32.277 2.127 0.534
Q Log10(Q) Ln(Q)
Total Bot 0.218741D-13 -13.660070 -31.453473
Total V=0 0.799686D+10 9.902920 22.802315
Vib (Bot) 0.286777D-23 -23.542456 -54.208509
Vib (V=0) 0.104841D+01 0.020533 0.047279
Electronic 0.100000D+01 0.000000 0.000000
Translational 0.583338D+07 6.765920 15.579107
Rotational 0.130757D+04 3.116466 7.175929
Cyclohexene
-------------------
- Thermochemistry -
-------------------
Temperature 298.150 Kelvin. Pressure 1.00000 Atm.
Atom 1 has atomic number 6 and mass 12.00000
Atom 2 has atomic number 6 and mass 12.00000
Atom 3 has atomic number 6 and mass 12.00000
Atom 4 has atomic number 6 and mass 12.00000
Atom 5 has atomic number 6 and mass 12.00000
Atom 6 has atomic number 6 and mass 12.00000
Atom 7 has atomic number 1 and mass 1.00783
Atom 8 has atomic number 1 and mass 1.00783
Atom 9 has atomic number 1 and mass 1.00783
Atom 10 has atomic number 1 and mass 1.00783
Atom 11 has atomic number 1 and mass 1.00783
Atom 12 has atomic number 1 and mass 1.00783
Atom 13 has atomic number 1 and mass 1.00783
Atom 14 has atomic number 1 and mass 1.00783
Atom 15 has atomic number 1 and mass 1.00783
Atom 16 has atomic number 1 and mass 1.00783
Molecular mass: 82.07825 amu.
Principal axes and moments of inertia in atomic units:
1 2 3
Eigenvalues -- 382.86444 398.79057 710.20540
X 1.00000 -0.00036 0.00243
Y 0.00036 1.00000 0.00000
Z -0.00243 0.00000 1.00000
This molecule is an asymmetric top.
Rotational symmetry number 1.
Rotational temperatures (Kelvin) 0.22623 0.21719 0.12196
Rotational constants (GHZ): 4.71379 4.52554 2.54115
Zero-point vibrational energy 385781.1 (Joules/Mol)
92.20390 (Kcal/Mol)
Warning -- explicit consideration of 5 degrees of freedom as
vibrations may cause significant error
Vibrational temperatures: 239.92 399.45 572.89 657.68 722.01
(Kelvin) 945.22 1055.81 1191.82 1201.92 1279.10
1319.06 1357.66 1440.98 1471.80 1518.66
1553.79 1593.32 1684.92 1687.80 1813.37
1844.89 1878.63 1967.99 2000.50 2003.28
2020.42 2068.67 2163.96 2174.34 2186.67
2202.50 2499.66 4320.70 4320.86 4358.16
4363.12 4385.90 4386.20 4426.91 4433.52
4524.87 4558.56
Zero-point correction= 0.146936 (Hartree/Particle)
Thermal correction to Energy= 0.152430
Thermal correction to Enthalpy= 0.153374
Thermal correction to Gibbs Free Energy= 0.118298
Sum of electronic and zero-point Energies= -234.501361
Sum of electronic and thermal Energies= -234.495867
Sum of electronic and thermal Enthalpies= -234.494923
Sum of electronic and thermal Free Energies= -234.529999
E (Thermal) CV S
KCal/Mol Cal/Mol-Kelvin Cal/Mol-Kelvin
Total 95.651 21.328 73.824
Electronic 0.000 0.000 0.000
Translational 0.889 2.981 39.129
Rotational 0.889 2.981 26.186
Vibrational 93.874 15.366 8.508
Vibration 1 0.624 1.883 2.472
Vibration 2 0.679 1.715 1.548
Vibration 3 0.764 1.474 0.969
Vibration 4 0.815 1.345 0.775
Vibration 5 0.857 1.246 0.654
Q Log10(Q) Ln(Q)
Total Bot 0.386237D-54 -54.413147 -125.290900
Total V=0 0.148833D+14 13.172698 30.331258
Vib (Bot) 0.112104D-66 -66.950377 -154.158941
Vib (Bot) 1 0.120980D+01 0.082715 0.190457
Vib (Bot) 2 0.693359D+00 -0.159042 -0.366207
Vib (Bot) 3 0.448223D+00 -0.348506 -0.802465
Vib (Bot) 4 0.372980D+00 -0.428314 -0.986230
Vib (Bot) 5 0.326984D+00 -0.485474 -1.117845
Vib (V=0) 0.431984D+01 0.635467 1.463217
Vib (V=0) 1 0.180905D+01 0.257452 0.592804
Vib (V=0) 2 0.135484D+01 0.131887 0.303681
Vib (V=0) 3 0.117149D+01 0.068740 0.158279
Vib (V=0) 4 0.112379D+01 0.050685 0.116707
Vib (V=0) 5 0.109743D+01 0.040375 0.092968
Electronic 0.100000D+01 0.000000 0.000000
Translational 0.292279D+08 7.465797 17.190634
Rotational 0.117878D+06 5.071433 11.677407
Diels-Alder TS (ethene + butadiene)
-------------------
- Thermochemistry -
-------------------
Temperature 298.150 Kelvin. Pressure 1.00000 Atm.
Atom 1 has atomic number 6 and mass 12.00000
Atom 2 has atomic number 6 and mass 12.00000
Atom 3 has atomic number 6 and mass 12.00000
Atom 4 has atomic number 6 and mass 12.00000
Atom 5 has atomic number 1 and mass 1.00783
Atom 6 has atomic number 1 and mass 1.00783
Atom 7 has atomic number 1 and mass 1.00783
Atom 8 has atomic number 1 and mass 1.00783
Atom 9 has atomic number 6 and mass 12.00000
Atom 10 has atomic number 1 and mass 1.00783
Atom 11 has atomic number 1 and mass 1.00783
Atom 12 has atomic number 6 and mass 12.00000
Atom 13 has atomic number 1 and mass 1.00783
Atom 14 has atomic number 1 and mass 1.00783
Atom 15 has atomic number 1 and mass 1.00783
Atom 16 has atomic number 1 and mass 1.00783
Molecular mass: 82.07825 amu.
Principal axes and moments of inertia in atomic units:
1 2 3
Eigenvalues -- 390.65162 396.49616 697.19520
X -0.00405 0.99999 -0.00002
Y 0.99998 0.00405 0.00428
Z -0.00428 0.00000 0.99999
This molecule is an asymmetric top.
Rotational symmetry number 1.
Rotational temperatures (Kelvin) 0.22172 0.21845 0.12423
Rotational constants (GHZ): 4.61982 4.55172 2.58857
1 imaginary frequencies ignored.
Zero-point vibrational energy 384722.1 (Joules/Mol)
91.95078 (Kcal/Mol)
Warning -- explicit consideration of 4 degrees of freedom as
vibrations may cause significant error
Vibrational temperatures: 264.90 600.41 641.67 763.05 1003.86
(Kelvin) 1007.93 1106.11 1179.43 1281.32 1360.73
1387.37 1402.12 1442.06 1516.38 1559.55
1590.21 1695.32 1729.49 1802.16 1814.30
1872.82 1949.23 1951.18 1982.64 2016.30
2053.85 2176.92 2188.89 2189.16 2219.42
2474.06 4318.56 4322.66 4381.22 4400.93
4422.02 4435.59 4436.88 4462.30 4553.22
4586.51
Zero-point correction= 0.146533 (Hartree/Particle)
Thermal correction to Energy= 0.151535
Thermal correction to Enthalpy= 0.152479
Thermal correction to Gibbs Free Energy= 0.118258
Sum of electronic and zero-point Energies= -234.492621
Sum of electronic and thermal Energies= -234.487619
Sum of electronic and thermal Enthalpies= -234.486675
Sum of electronic and thermal Free Energies= -234.520896
E (Thermal) CV S
KCal/Mol Cal/Mol-Kelvin Cal/Mol-Kelvin
Total 95.090 19.620 72.025
Electronic 0.000 0.000 0.000
Translational 0.889 2.981 39.129
Rotational 0.889 2.981 26.182
Vibrational 93.312 13.658 6.713
Vibration 1 0.631 1.861 2.286
Vibration 2 0.780 1.433 0.901
Vibration 3 0.805 1.370 0.808
Vibration 4 0.885 1.183 0.586
Q Log10(Q) Ln(Q)
Total Bot 0.402907D-54 -54.394796 -125.248646
Total V=0 0.101278D+14 13.005516 29.946308
Vib (Bot) 0.117184D-66 -66.931131 -154.114624
Vib (Bot) 1 0.108931D+01 0.037153 0.085547
Vib (Bot) 2 0.421637D+00 -0.375062 -0.863611
Vib (Bot) 3 0.385764D+00 -0.413678 -0.952528
Vib (Bot) 4 0.301456D+00 -0.520777 -1.199132
Vib (V=0) 0.294565D+01 0.469181 1.080329
Vib (V=0) 1 0.169858D+01 0.230087 0.529795
Vib (V=0) 2 0.115405D+01 0.062224 0.143275
Vib (V=0) 3 0.113152D+01 0.053661 0.123560
Vib (V=0) 4 0.108385D+01 0.034967 0.080515
Electronic 0.100000D+01 0.000000 0.000000
Translational 0.292279D+08 7.465797 17.190634
Rotational 0.117635D+06 5.070538 11.675345
TS cis-trans isomerisation of butadiene
-------------------
- Thermochemistry -
-------------------
Temperature 298.150 Kelvin. Pressure 1.00000 Atm.
Atom 1 has atomic number 6 and mass 12.00000
Atom 2 has atomic number 1 and mass 1.00783
Atom 3 has atomic number 1 and mass 1.00783
Atom 4 has atomic number 6 and mass 12.00000
Atom 5 has atomic number 1 and mass 1.00783
Atom 6 has atomic number 6 and mass 12.00000
Atom 7 has atomic number 1 and mass 1.00783
Atom 8 has atomic number 6 and mass 12.00000
Atom 9 has atomic number 1 and mass 1.00783
Atom 10 has atomic number 1 and mass 1.00783
Molecular mass: 54.04695 amu.
Principal axes and moments of inertia in atomic units:
1 2 3
Eigenvalues -- 64.34089 395.43274 403.53642
X 0.99999 0.00000 0.00481
Y 0.00000 1.00000 0.00000
Z -0.00481 0.00000 0.99999
This molecule is an asymmetric top.
Rotational symmetry number 1.
Rotational temperatures (Kelvin) 1.34617 0.21904 0.21464
Rotational constants (GHZ): 28.04968 4.56397 4.47231
1 imaginary frequencies ignored.
Zero-point vibrational energy 221831.2 (Joules/Mol)
53.01894 (Kcal/Mol)
Warning -- explicit consideration of 2 degrees of freedom as
vibrations may cause significant error
Vibrational temperatures: 471.46 512.53 941.15 1005.54 1264.67
(Kelvin) 1367.27 1371.51 1450.44 1479.44 1526.76
1646.97 1897.61 1905.96 2064.65 2121.01
2439.98 2496.02 4500.85 4502.12 4536.40
4538.78 4659.48 4659.69
Zero-point correction= 0.084491 (Hartree/Particle)
Thermal correction to Energy= 0.088613
Thermal correction to Enthalpy= 0.089557
Thermal correction to Gibbs Free Energy= 0.058399
Sum of electronic and zero-point Energies= -155.905140
Sum of electronic and thermal Energies= -155.901018
Sum of electronic and thermal Enthalpies= -155.900074
Sum of electronic and thermal Free Energies= -155.931232
E (Thermal) CV S
KCal/Mol Cal/Mol-Kelvin Cal/Mol-Kelvin
Total 55.605 14.087 65.577
Electronic 0.000 0.000 0.000
Translational 0.889 2.981 37.884
Rotational 0.889 2.981 23.844
Vibrational 53.828 8.125 3.849
Vibration 1 0.711 1.620 1.272
Vibration 2 0.732 1.562 1.139
Q Log10(Q) Ln(Q)
Total Bot 0.137496D-26 -26.861711 -61.851376
Total V=0 0.100322D+13 12.001395 27.634234
Vib (Bot) 0.242722D-38 -38.614891 -88.914073
Vib (Bot) 1 0.571023D+00 -0.243347 -0.560327
Vib (Bot) 2 0.515824D+00 -0.287498 -0.661990
Vib (V=0) 0.177099D+01 0.248216 0.571538
Vib (V=0) 1 0.125899D+01 0.100022 0.230310
Vib (V=0) 2 0.121838D+01 0.085784 0.197525
Electronic 0.100000D+01 0.000000 0.000000
Translational 0.156175D+08 7.193612 16.563904
Rotational 0.362717D+05 4.559568 10.498792
Cyclohexadiene
-------------------
- Thermochemistry -
-------------------
Temperature 298.150 Kelvin. Pressure 1.00000 Atm.
Atom 1 has atomic number 6 and mass 12.00000
Atom 2 has atomic number 6 and mass 12.00000
Atom 3 has atomic number 6 and mass 12.00000
Atom 4 has atomic number 6 and mass 12.00000
Atom 5 has atomic number 6 and mass 12.00000
Atom 6 has atomic number 6 and mass 12.00000
Atom 7 has atomic number 1 and mass 1.00783
Atom 8 has atomic number 1 and mass 1.00783
Atom 9 has atomic number 1 and mass 1.00783
Atom 10 has atomic number 1 and mass 1.00783
Atom 11 has atomic number 1 and mass 1.00783
Atom 12 has atomic number 1 and mass 1.00783
Atom 13 has atomic number 1 and mass 1.00783
Atom 14 has atomic number 1 and mass 1.00783
Molecular mass: 80.06260 amu.
Principal axes and moments of inertia in atomic units:
1 2 3
Eigenvalues -- 356.90758 357.72459 674.59638
X 0.00000 0.07914 0.99686
Y 0.00000 0.99686 -0.07914
Z 1.00000 0.00000 0.00000
This molecule is an asymmetric top.
Rotational symmetry number 2.
Rotational temperatures (Kelvin) 0.24268 0.24212 0.12839
Rotational constants (GHZ): 5.05661 5.04506 2.67529
Zero-point vibrational energy 321551.0 (Joules/Mol)
76.85252 (Kcal/Mol)
Warning -- explicit consideration of 5 degrees of freedom as
vibrations may cause significant error
Vibrational temperatures: 271.58 432.66 690.69 747.18 823.16
(Kelvin) 970.16 1095.79 1124.70 1232.49 1346.28
1394.61 1401.20 1425.07 1453.15 1504.93
1545.18 1693.23 1714.55 1737.78 1832.00
1956.78 1970.33 2028.21 2083.33 2131.75
2148.68 2383.96 2475.92 4286.86 4302.08
4423.71 4423.94 4552.47 4562.38 4583.62
4596.84
Zero-point correction= 0.122472 (Hartree/Particle)
Thermal correction to Energy= 0.127660
Thermal correction to Enthalpy= 0.128605
Thermal correction to Gibbs Free Energy= 0.094865
Sum of electronic and zero-point Energies= -233.308495
Sum of electronic and thermal Energies= -233.303307
Sum of electronic and thermal Enthalpies= -233.302363
Sum of electronic and thermal Free Energies= -233.336102
E (Thermal) CV S
KCal/Mol Cal/Mol-Kelvin Cal/Mol-Kelvin
Total 80.108 20.037 71.010
Electronic 0.000 0.000 0.000
Translational 0.889 2.981 39.055
Rotational 0.889 2.981 24.580
Vibrational 78.331 14.075 7.375
Vibration 1 0.633 1.855 2.240
Vibration 2 0.693 1.672 1.413
Vibration 3 0.836 1.294 0.710
Vibration 4 0.874 1.207 0.612
Vibration 5 0.928 1.092 0.500
Q Log10(Q) Ln(Q)
Total Bot 0.231765D-43 -43.634952 -100.473190
Total V=0 0.499197D+13 12.698272 29.238853
Vib (Bot) 0.156696D-55 -55.804942 -128.495628
Vib (Bot) 1 0.106078D+01 0.025626 0.059005
Vib (Bot) 2 0.632159D+00 -0.199174 -0.458615
Vib (Bot) 3 0.348372D+00 -0.457957 -1.054485
Vib (Bot) 4 0.311012D+00 -0.507223 -1.167924
Vib (Bot) 5 0.268439D+00 -0.571155 -1.315132
Vib (V=0) 0.337506D+01 0.528282 1.216414
Vib (V=0) 1 0.167271D+01 0.223421 0.514447
Vib (V=0) 2 0.130599D+01 0.115941 0.266964
Vib (V=0) 3 0.110940D+01 0.045086 0.103815
Vib (V=0) 4 0.108884D+01 0.036963 0.085110
Vib (V=0) 5 0.106750D+01 0.028369 0.065322
Electronic 0.100000D+01 0.000000 0.000000
Translational 0.281579D+08 7.449600 17.153337
Rotational 0.525280D+05 4.720391 10.869101
Maleic anhydride
-------------------
- Thermochemistry -
-------------------
Temperature 298.150 Kelvin. Pressure 1.00000 Atm.
Atom 1 has atomic number 6 and mass 12.00000
Atom 2 has atomic number 6 and mass 12.00000
Atom 3 has atomic number 6 and mass 12.00000
Atom 4 has atomic number 6 and mass 12.00000
Atom 5 has atomic number 1 and mass 1.00783
Atom 6 has atomic number 1 and mass 1.00783
Atom 7 has atomic number 8 and mass 15.99491
Atom 8 has atomic number 8 and mass 15.99491
Atom 9 has atomic number 8 and mass 15.99491
Molecular mass: 98.00039 amu.
Principal axes and moments of inertia in atomic units:
1 2 3
Eigenvalues -- 263.77954 737.061191000.84048
X 0.00000 -0.00232 1.00000
Y 0.00000 1.00000 0.00232
Z 1.00000 0.00000 0.00000
This molecule is an asymmetric top.
Rotational symmetry number 1.
Rotational temperatures (Kelvin) 0.32836 0.11751 0.08654
Rotational constants (GHZ): 6.84185 2.44856 1.80323
Zero-point vibrational energy 146573.5 (Joules/Mol)
35.03191 (Kcal/Mol)
Warning -- explicit consideration of 4 degrees of freedom as
vibrations may cause significant error
Vibrational temperatures: 241.14 379.73 575.44 803.49 915.86
(Kelvin) 920.78 1011.02 1095.91 1227.29 1261.02
1311.56 1408.07 1529.15 1557.06 1836.84
1925.08 2395.56 2686.92 2775.96 4685.23
4714.32
Zero-point correction= 0.055827 (Hartree/Particle)
Thermal correction to Energy= 0.061013
Thermal correction to Enthalpy= 0.061957
Thermal correction to Gibbs Free Energy= 0.026753
Sum of electronic and zero-point Energies= -379.236895
Sum of electronic and thermal Energies= -379.231709
Sum of electronic and thermal Enthalpies= -379.230765
Sum of electronic and thermal Free Energies= -379.265969
E (Thermal) CV S
KCal/Mol Cal/Mol-Kelvin Cal/Mol-Kelvin
Total 38.286 18.580 74.093
Electronic 0.000 0.000 0.000
Translational 0.889 2.981 39.658
Rotational 0.889 2.981 26.767
Vibrational 36.508 12.619 7.668
Vibration 1 0.624 1.882 2.462
Vibration 2 0.670 1.739 1.636
Vibration 3 0.766 1.470 0.963
Vibration 4 0.914 1.121 0.527
Q Log10(Q) Ln(Q)
Total Bot 0.495023D-12 -12.305374 -28.334172
Total V=0 0.236135D+14 13.373160 30.792839
Vib (Bot) 0.822105D-25 -25.085073 -57.760514
Vib (Bot) 1 0.120335D+01 0.080391 0.185107
Vib (Bot) 2 0.734511D+00 -0.134002 -0.308550
Vib (Bot) 3 0.445662D+00 -0.350995 -0.808195
Vib (Bot) 4 0.278725D+00 -0.554825 -1.277531
Vib (V=0) 0.392159D+01 0.593462 1.366497
Vib (V=0) 1 0.180309D+01 0.256018 0.589502
Vib (V=0) 2 0.138854D+01 0.142559 0.328254
Vib (V=0) 3 0.116979D+01 0.068107 0.156821
Vib (V=0) 4 0.107244D+01 0.030373 0.069936
Electronic 0.100000D+01 0.000000 0.000000
Translational 0.381326D+08 7.581297 17.456581
Rotational 0.157907D+06 5.198401 11.969761
EXO TS
-------------------
- Thermochemistry -
-------------------
Temperature 298.150 Kelvin. Pressure 1.00000 Atm.
Atom 1 has atomic number 6 and mass 12.00000
Atom 2 has atomic number 6 and mass 12.00000
Atom 3 has atomic number 6 and mass 12.00000
Atom 4 has atomic number 6 and mass 12.00000
Atom 5 has atomic number 6 and mass 12.00000
Atom 6 has atomic number 6 and mass 12.00000
Atom 7 has atomic number 1 and mass 1.00783
Atom 8 has atomic number 1 and mass 1.00783
Atom 9 has atomic number 1 and mass 1.00783
Atom 10 has atomic number 1 and mass 1.00783
Atom 11 has atomic number 1 and mass 1.00783
Atom 12 has atomic number 1 and mass 1.00783
Atom 13 has atomic number 1 and mass 1.00783
Atom 14 has atomic number 1 and mass 1.00783
Atom 15 has atomic number 6 and mass 12.00000
Atom 16 has atomic number 6 and mass 12.00000
Atom 17 has atomic number 6 and mass 12.00000
Atom 18 has atomic number 6 and mass 12.00000
Atom 19 has atomic number 1 and mass 1.00783
Atom 20 has atomic number 1 and mass 1.00783
Atom 21 has atomic number 8 and mass 15.99491
Atom 22 has atomic number 8 and mass 15.99491
Atom 23 has atomic number 8 and mass 15.99491
Molecular mass: 178.06299 amu.
Principal axes and moments of inertia in atomic units:
1 2 3
Eigenvalues -- 1508.171772101.216802729.27762
X 1.00000 0.00002 -0.00009
Y -0.00002 1.00000 -0.00002
Z 0.00009 0.00002 1.00000
This molecule is an asymmetric top.
Rotational symmetry number 1.
Warning -- assumption of classical behavior for rotation
may cause significant error
Rotational temperatures (Kelvin) 0.05743 0.04122 0.03174
Rotational constants (GHZ): 1.19664 0.85890 0.66125
1 imaginary frequencies ignored.
Zero-point vibrational energy 474804.2 (Joules/Mol)
113.48092 (Kcal/Mol)
Warning -- explicit consideration of 14 degrees of freedom as
vibrations may cause significant error
Vibrational temperatures: 75.40 157.95 194.90 232.93 260.14
(Kelvin) 319.64 343.03 524.14 585.67 596.55
759.66 804.38 851.71 865.88 903.08
1018.93 1053.29 1071.03 1099.89 1182.37
1202.19 1206.12 1252.56 1283.22 1290.07
1307.14 1371.92 1410.74 1414.85 1470.69
1473.37 1506.59 1531.42 1540.22 1597.78
1691.51 1697.88 1760.71 1821.71 1840.78
1884.15 1924.01 1955.70 2010.44 2049.23
2117.02 2167.65 2191.78 2228.24 2277.04
2656.71 2740.73 4365.83 4389.33 4460.27
4479.04 4562.89 4578.07 4592.57 4602.55
4693.59 4712.63
Zero-point correction= 0.180843 (Hartree/Particle)
Thermal correction to Energy= 0.191209
Thermal correction to Enthalpy= 0.192153
Thermal correction to Gibbs Free Energy= 0.144577
Sum of electronic and zero-point Energies= -612.513548
Sum of electronic and thermal Energies= -612.503182
Sum of electronic and thermal Enthalpies= -612.502238
Sum of electronic and thermal Free Energies= -612.549814
E (Thermal) CV S
KCal/Mol Cal/Mol-Kelvin Cal/Mol-Kelvin
Total 119.985 40.910 100.132
Electronic 0.000 0.000 0.000
Translational 0.889 2.981 41.438
Rotational 0.889 2.981 30.537
Vibrational 118.208 34.948 28.157
Vibration 1 0.596 1.977 4.724
Vibration 2 0.606 1.941 3.273
Vibration 3 0.613 1.918 2.867
Vibration 4 0.622 1.889 2.528
Vibration 5 0.630 1.866 2.320
Vibration 6 0.648 1.807 1.941
Vibration 7 0.656 1.782 1.815
Vibration 8 0.738 1.546 1.104
Vibration 9 0.772 1.455 0.937
Vibration 10 0.778 1.438 0.910
Vibration 11 0.883 1.188 0.592
Vibration 12 0.915 1.120 0.526
Vibration 13 0.949 1.049 0.464
Vibration 14 0.960 1.028 0.447
Q Log10(Q) Ln(Q)
Total Bot 0.315644D-66 -66.500803 -153.123758
Total V=0 0.479947D+17 16.681193 38.409867
Vib (Bot) 0.321033D-80 -80.493450 -185.343018
Vib (Bot) 1 0.394368D+01 0.595902 1.372115
Vib (Bot) 2 0.186569D+01 0.270840 0.623633
Vib (Bot) 3 0.150289D+01 0.176928 0.407392
Vib (Bot) 4 0.124800D+01 0.096216 0.221545
Vib (Bot) 5 0.111054D+01 0.045536 0.104850
Vib (Bot) 6 0.889545D+00 -0.050832 -0.117045
Vib (Bot) 7 0.823012D+00 -0.084594 -0.194784
Vib (Bot) 8 0.501695D+00 -0.299560 -0.689762
Vib (Bot) 9 0.435589D+00 -0.360923 -0.831056
Vib (Bot) 10 0.425229D+00 -0.371378 -0.855128
Vib (Bot) 11 0.303466D+00 -0.517890 -1.192486
Vib (Bot) 12 0.278250D+00 -0.555565 -1.279237
Vib (Bot) 13 0.254324D+00 -0.594613 -1.369147
Vib (Bot) 14 0.247652D+00 -0.606157 -1.395729
Vib (V=0) 0.488142D+03 2.688546 6.190607
Vib (V=0) 1 0.447525D+01 0.650818 1.498563
Vib (V=0) 2 0.243153D+01 0.385880 0.888521
Vib (V=0) 3 0.208388D+01 0.318874 0.734233
Vib (V=0) 4 0.184444D+01 0.265864 0.612174
Vib (V=0) 5 0.171791D+01 0.235001 0.541109
Vib (V=0) 6 0.152044D+01 0.181968 0.418997
Vib (V=0) 7 0.146299D+01 0.165241 0.380482
Vib (V=0) 8 0.120831D+01 0.082177 0.189220
Vib (V=0) 9 0.116313D+01 0.065627 0.151112
Vib (V=0) 10 0.115637D+01 0.063096 0.145284
Vib (V=0) 11 0.108489D+01 0.035384 0.081475
Vib (V=0) 12 0.107221D+01 0.030279 0.069721
Vib (V=0) 13 0.106096D+01 0.025701 0.059178
Vib (V=0) 14 0.105797D+01 0.024474 0.056353
Electronic 0.100000D+01 0.000000 0.000000
Translational 0.933933D+08 7.970316 18.352330
Rotational 0.105276D+07 6.022331 13.866930
EXO product
-------------------
- Thermochemistry -
-------------------
Temperature 298.150 Kelvin. Pressure 1.00000 Atm.
Atom 1 has atomic number 6 and mass 12.00000
Atom 2 has atomic number 6 and mass 12.00000
Atom 3 has atomic number 6 and mass 12.00000
Atom 4 has atomic number 6 and mass 12.00000
Atom 5 has atomic number 1 and mass 1.00783
Atom 6 has atomic number 1 and mass 1.00783
Atom 7 has atomic number 6 and mass 12.00000
Atom 8 has atomic number 1 and mass 1.00783
Atom 9 has atomic number 6 and mass 12.00000
Atom 10 has atomic number 1 and mass 1.00783
Atom 11 has atomic number 1 and mass 1.00783
Atom 12 has atomic number 1 and mass 1.00783
Atom 13 has atomic number 6 and mass 12.00000
Atom 14 has atomic number 1 and mass 1.00783
Atom 15 has atomic number 1 and mass 1.00783
Atom 16 has atomic number 6 and mass 12.00000
Atom 17 has atomic number 1 and mass 1.00783
Atom 18 has atomic number 1 and mass 1.00783
Atom 19 has atomic number 6 and mass 12.00000
Atom 20 has atomic number 6 and mass 12.00000
Atom 21 has atomic number 8 and mass 15.99491
Atom 22 has atomic number 8 and mass 15.99491
Atom 23 has atomic number 8 and mass 15.99491
Molecular mass: 178.06299 amu.
Principal axes and moments of inertia in atomic units:
1 2 3
Eigenvalues -- 1404.985302019.548802724.37272
X 0.99996 -0.00003 -0.00913
Y 0.00003 1.00000 0.00001
Z 0.00913 -0.00001 0.99996
This molecule is an asymmetric top.
Rotational symmetry number 1.
Warning -- assumption of classical behavior for rotation
may cause significant error
Rotational temperatures (Kelvin) 0.06165 0.04289 0.03179
Rotational constants (GHZ): 1.28453 0.89364 0.66244
Zero-point vibrational energy 487669.5 (Joules/Mol)
116.55580 (Kcal/Mol)
Warning -- explicit consideration of 12 degrees of freedom as
vibrations may cause significant error
Vibrational temperatures: 83.42 203.53 227.65 266.91 345.00
(Kelvin) 457.29 542.07 553.03 631.16 751.67
850.93 857.37 898.54 913.20 962.29
998.14 1066.07 1130.74 1161.95 1212.49
1232.36 1257.93 1321.80 1330.14 1379.87
1398.08 1403.18 1442.13 1469.83 1509.39
1534.24 1584.28 1594.30 1649.30 1683.88
1732.65 1777.09 1798.14 1808.13 1819.08
1843.55 1848.62 1906.53 1924.37 1939.09
1956.22 2000.48 2025.73 2161.14 2188.63
2429.81 2691.33 2790.54 4385.71 4403.54
4418.63 4435.16 4455.32 4479.84 4480.15
4483.74 4594.31 4624.46
Zero-point correction= 0.185743 (Hartree/Particle)
Thermal correction to Energy= 0.195210
Thermal correction to Enthalpy= 0.196154
Thermal correction to Gibbs Free Energy= 0.150672
Sum of electronic and zero-point Energies= -612.587025
Sum of electronic and thermal Energies= -612.577559
Sum of electronic and thermal Enthalpies= -612.576615
Sum of electronic and thermal Free Energies= -612.622097
E (Thermal) CV S
KCal/Mol Cal/Mol-Kelvin Cal/Mol-Kelvin
Total 122.496 38.858 95.725
Electronic 0.000 0.000 0.000
Translational 0.889 2.981 41.438
Rotational 0.889 2.981 30.426
Vibrational 120.719 32.896 23.862
Vibration 1 0.596 1.974 4.525
Vibration 2 0.615 1.912 2.784
Vibration 3 0.621 1.893 2.571
Vibration 4 0.632 1.860 2.272
Vibration 5 0.657 1.780 1.804
Vibration 6 0.704 1.640 1.321
Vibration 7 0.747 1.520 1.052
Vibration 8 0.753 1.504 1.022
Vibration 9 0.799 1.386 0.831
Vibration 10 0.877 1.200 0.604
Vibration 11 0.949 1.050 0.465
Vibration 12 0.954 1.041 0.457
Q Log10(Q) Ln(Q)
Total Bot 0.496410D-69 -69.304159 -159.578724
Total V=0 0.135435D+17 16.131731 37.144684
Vib (Bot) 0.534050D-83 -83.272418 -191.741828
Vib (Bot) 1 0.356247D+01 0.551751 1.270454
Vib (Bot) 2 0.143687D+01 0.157417 0.362467
Vib (Bot) 3 0.127838D+01 0.106660 0.245595
Vib (Bot) 4 0.108062D+01 0.033671 0.077531
Vib (Bot) 5 0.817801D+00 -0.087352 -0.201136
Vib (Bot) 6 0.592208D+00 -0.227526 -0.523898
Vib (Bot) 7 0.480983D+00 -0.317871 -0.731924
Vib (Bot) 8 0.468943D+00 -0.328880 -0.757273
Vib (Bot) 9 0.394488D+00 -0.403966 -0.930166
Vib (Bot) 10 0.308271D+00 -0.511067 -1.176775
Vib (Bot) 11 0.254696D+00 -0.593978 -1.367685
Vib (Bot) 12 0.251631D+00 -0.599236 -1.379791
Vib (V=0) 0.145704D+03 2.163473 4.981580
Vib (V=0) 1 0.409739D+01 0.612507 1.410349
Vib (V=0) 2 0.202138D+01 0.305648 0.703780
Vib (V=0) 3 0.187268D+01 0.272464 0.627372
Vib (V=0) 4 0.169068D+01 0.228063 0.525134
Vib (V=0) 5 0.145854D+01 0.163918 0.377436
Vib (V=0) 6 0.127505D+01 0.105529 0.242989
Vib (V=0) 7 0.119379D+01 0.076928 0.177133
Vib (V=0) 8 0.118550D+01 0.073901 0.170163
Vib (V=0) 9 0.113688D+01 0.055716 0.128291
Vib (V=0) 10 0.108739D+01 0.036387 0.083784
Vib (V=0) 11 0.106113D+01 0.025770 0.059337
Vib (V=0) 12 0.105975D+01 0.025203 0.058031
Electronic 0.100000D+01 0.000000 0.000000
Translational 0.933933D+08 7.970316 18.352330
Rotational 0.995274D+06 5.997943 13.810774
References and literature
- ↑ Self-replicating Cope Rearrangements. Michigan State University. https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/bullvalene.htm
- ↑ Fukui, Kenichi; Yonezawa, Teijiro; Shingu, Haruo (1952). "A Molecular Orbital Theory of Reactivity in Aromatic Hydrocarbons". The Journal of Chemical Physics 20 (4): 722. Bibcode:1952JChPh..20..722F. doi:10.1063/1.1700523.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 K.Kahn. Molecular Orbitals in Conjugated Systems. UC Santa Barbara. http://people.chem.ucsb.edu/kahn/kalju/chem109C/DielsAlder.html
- ↑ http://chemwiki.ucdavis.edu/Theoretical_Chemistry/Chemical_Bonding/General_Principles_of_Chemical_Bonding/Covalent_Bond_Distance,_Radius_and_van_der_Waals_Radius#Van_der_Waals_Radius
- ↑ 5.0 5.1 http://www.chem.wayne.edu/schlegel/Pub_folder/108.pdf
- ↑ 6.0 6.1 T.L. Gilchrist, R.C. Storr. Organic reactions and orbital symmetry. Cambridge University Press. 1979.
- ↑ 7.0 7.1 7.2 A. Arrieta, F. P. Cossío. Direct Evaluation of Secondary Orbital Interactions in the Diels-Alder Reaction between Cyclopentadiene and Maleic Anhydride. J. Org. Chem. 2001, 66, 6178-6180. Accessible online: http://pubs.acs.org/doi/pdf/10.1021/jo0158478
